Difference between revisions of "RibE"
| Line 50: | Line 50: | ||
| + | |||
| + | = Categories containing this gene/protein = | ||
| + | {{SubtiWiki category|[[biosynthesis of cofactors]]}} | ||
=The protein= | =The protein= | ||
Revision as of 19:20, 30 November 2010
- Description: riboflavin synthase (alpha subunit)
| Gene name | ribE |
| Synonyms | ribB |
| Essential | no |
| Product | riboflavin synthase (alpha subunit) |
| Function | riboflavin biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Riboflavin / FAD | |
| MW, pI | 23 kDa, 5.845 |
| Gene length, protein length | 645 bp, 215 aa |
| Immediate neighbours | ribA, ribD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 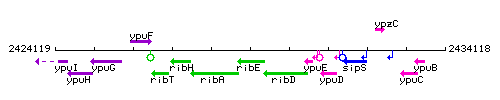
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU23270
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
Categories containing this gene/protein
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 6,7-dimethyl-8-(1-D-ribityl)lumazine = riboflavin + 4-(1-D-ribitylamino)-5-amino-2,6-dihydroxypyrimidine (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 1I8D (from E. coli, 36% identity, 59% similarity) PubMed, , 1RVV (the RibE)3-(RibH)60 lumazine synthase/riboflavin synthase complex) PubMed
- UniProt: P16440
- KEGG entry: [3]
- E.C. number: 2.5.1.9
Additional information
Expression and regulation
- Regulatory mechanism: FMN-box: riboswitch, mediates termination/ antitermination control of the operon, in the absence of FMN: antitermination, in the presence of FMN: termination PubMed
- Additional information:
Biological materials
- Mutant: GP203 (erm), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References