Difference between revisions of "PdhA"
(→Reviews) |
|||
| Line 159: | Line 159: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed> 19476487 9655937 2227213 6805383 </pubmed> | + | <pubmed> 19476487 9655937 2227213 6805383 24798336 </pubmed> |
| + | |||
==Original publications== | ==Original publications== | ||
<pubmed>9352926, 20525796, 12850135 6414463 11976308 20081037 15378759 24825009</pubmed> | <pubmed>9352926, 20525796, 12850135 6414463 11976308 20081037 15378759 24825009</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:51, 28 May 2014
- Description: pyruvate dehydrogenase (E1 alpha subunit), required for Z-ring assembly in a pyruvate-dependent manner
| Gene name | pdhA |
| Synonyms | aceA |
| Essential | yes PubMed, no PubMed |
| Product | pyruvate dehydrogenase (E1 alpha subunit) |
| Function | links glycolysis and TCA cycle |
| Gene expression levels in SubtiExpress: pdhA | |
| Interactions involving this protein in SubtInteract: PdhA | |
| Metabolic function and regulation of this protein in SubtiPathways: pdhA | |
| MW, pI | 41 kDa, 5.837 |
| Gene length, protein length | 1113 bp, 371 aa |
| Immediate neighbours | ykyA, pdhB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 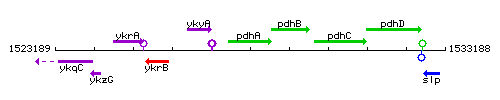
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, essential genes, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14580
Phenotypes of a mutant
- pdhA is essential according to Kobayashi et al. PubMed
- the mutant grows slowly but is viable PubMed
- depletion of pdhA and deletion of ezrA have a strong synthetic defect in cell division PubMed
Database entries
- BsubCyc: BSU14580
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Pyruvate + [dihydrolipoyllysine-residue acetyltransferase] lipoyllysine = [dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine + CO2 (according to Swiss-Prot)
- Protein family:
Extended information on the protein
- Kinetic information: Michaelis-Menten PubMed
- Modification:
- Cofactors:
- thiamine pyrophosphate
- Effectors of protein activity:
- Inhibited thiamine 2-thiothiazolone diphosphate and NADH PubMed
- Low sensibility to NADPH
- Localization:
- colocalizes with the nucleoid (depending on the availability of pyruvate) PubMed
Database entries
- BsubCyc: BSU14580
- Structure: 1W88 (E1 in complex with subunit binding domain of E2, Geobacillus stearothermophilus)
- UniProt: P21881
- KEGG entry: [3]
- E.C. number: 1.2.4.1
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- stringent response: due to presence of guanine at +1 position of the transcript PubMed
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 5117 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 18311 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 4425 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 5452 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 7055 PubMed
Biological materials
- Mutant:
- Expression vector:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Arthur Aronson, Purdue University, West Lafayette, USA homepage
Your additional remarks
References
Reviews
Original publications