PtkA
- Description: protein tyrosine kinase
| Gene name | ptkA |
| Synonyms | ywqD |
| Essential | no |
| Product | protein tyrosine kinase |
| Function | protein phosphorylation |
| Gene expression levels in SubtiExpress: ptkA | |
| Interactions involving this protein in SubtInteract: PtkA | |
| MW, pI | 25 kDa, 9.628 |
| Gene length, protein length | 711 bp, 237 aa |
| Immediate neighbours | ptpZ, tkmA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed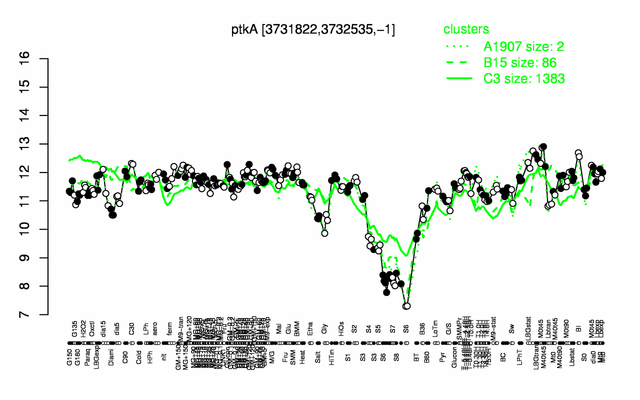
| |
Contents
Categories containing this gene/protein
biofilm formation, protein modification, membrane proteins
This gene is a member of the following regulons
AbrB regulon, DegU regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU36250
Phenotypes of a mutant
- Accumulation of extra chromosome equivalents PubMed
- Defect in biofilm formation, this involves the kinase activity, but the target protein is unknown PubMed
Database entries
- BsubCyc: BSU36250
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + a [protein]-L-tyrosine = ADP + a [protein]-L-tyrosine phosphate (according to Swiss-Prot), autophosphorylation, phosphorylation of Ugd, TuaD, Ssb, SsbB
- Protein family: BY-kinase, see the Bacterial Protein Tyrosine Kinase Database)
- Paralogous protein(s): EpsB
Extended information on the protein
- Kinetic information:
- Domains: single BY-kinase domain
- Modification: autophosphorylation at residues Y225, Y227 and Y228 (primary site) PubMed, dephosphorylated by PtpZ PubMed
- Cofactors: ATP
- Effectors of protein activity: TkmA - transmembrane modulator, activates PtkA autophosphorylation and substrate phosphorylation PubMed
Database entries
- BsubCyc: BSU36250
- Structure: 2VED (CapB, the homolog in Staphylococcus aureus)
- UniProt: P96716
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- KO strain created with pMUTIN-2, available from Ivan Mijakovic
- GP1520 (spc), available in Jörg Stülke's lab
- GP1544 (ermC), available in Jörg Stülke's lab
- GP1587 (cat) , available in Jörg Stülke's lab
- GP1521 epsB (aphA3) ptkA (spc) double mutant available in Jörg Stülke's lab
- GP1529 tkmA-ptkA::spc available in Jörg Stülke's lab
- GP1610 (ptkA-ptpZ, spc), available in Jörg Stülke's lab
- Expression vector: pQE-30, N-terminally 6xHis-tagged, available from Ivan Mijakovic
- lacZ fusion: in a KO strain created with pMUTIN-2, available from Ivan Mijakovic
- GFP fusion: CFP-fusion, available from Ivan Mijakovic
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Ivan Mijakovic, Thiverval-Grignon, France
Your additional remarks
References
Reviews
Ivan Mijakovic, Josef Deutscher
Protein-tyrosine phosphorylation in Bacillus subtilis: a 10-year retrospective.
Front Microbiol: 2015, 6;18
[PubMed:25667587]
[WorldCat.org]
[DOI]
(P e)
Jan Gerwig, Jörg Stülke
Far from being well understood: multiple protein phosphorylation events control cell differentiation in Bacillus subtilis at different levels.
Front Microbiol: 2014, 5;704
[PubMed:25540643]
[WorldCat.org]
[DOI]
(P e)
Joseph D Chao, Dennis Wong, Yossef Av-Gay
Microbial protein-tyrosine kinases.
J Biol Chem: 2014, 289(14);9463-72
[PubMed:24554699]
[WorldCat.org]
[DOI]
(I p)
Jörg Stülke
More than just activity control: phosphorylation may control all aspects of a protein's properties.
Mol Microbiol: 2010, 77(2);273-5
[PubMed:20497498]
[WorldCat.org]
[DOI]
(I p)
Original publications