Hom
Revision as of 14:08, 17 April 2014 by 134.76.70.252 (talk)
- Description: homoserine dehydrogenase (NADPH)
| Gene name | hom |
| Synonyms | |
| Essential | no |
| Product | homoserine dehydrogenase (NADPH) |
| Function | biosynthesis of methionine and threonine |
| Gene expression levels in SubtiExpress: hom | |
| Metabolic function and regulation of this protein in SubtiPathways: hom | |
| MW, pI | 47 kDa, 4.9 |
| Gene length, protein length | 1299 bp, 433 aa |
| Immediate neighbours | thrC, yutH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 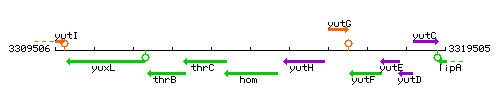
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed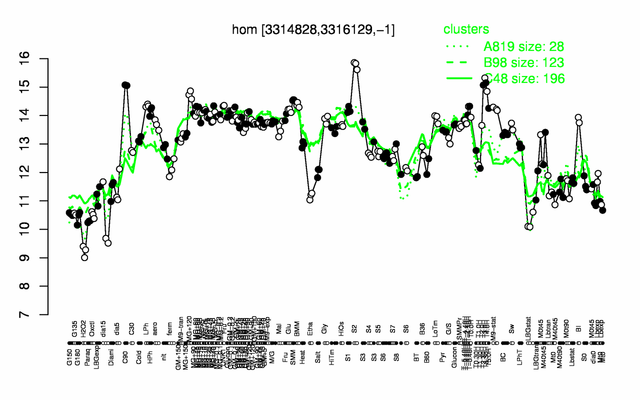
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, membrane proteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU32260
Phenotypes of a mutant
Database entries
- BsubCyc: BSU32260
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-homoserine + NAD(P)+ = L-aspartate 4-semialdehyde + NAD(P)H (according to Swiss-Prot)
- Protein family: homoserine dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity: subject to feedback inhibition PubMed
- Localization:
- membrane associated PubMed
Database entries
- BsubCyc: BSU32260
- Structure: 2EJW (from Thermus thermophilus hb8, 37% identity, 57% similarity)
- UniProt: P19582
- KEGG entry: [2]
- E.C. number: 1.1.1.3
Additional information
Expression and regulation
- Regulation:
- Additional information:
- subject to feedback inhibition PubMed
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 2167 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 1723 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 3436 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 2047 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 3239 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References