Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' repressor of the glycolytic ''[[gapA]]'' operon<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''cggR'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''yvbQ '' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || no |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || central glycolytic genes regulator |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || transcriptional regulator |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 37,2 kDa,5.68 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1020 bp, 340 amino acids |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[araE]]'', ''[[gapA]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:cggR_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 30: | Line 30: | ||
<br/><br/> | <br/><br/> | ||
| + | |||
=The gene= | =The gene= | ||
| Line 35: | Line 36: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Coordinates:''' | + | * '''Coordinates:''' 3481786 - 3482805 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| Line 41: | Line 42: | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/cggR-gapA-pgk-tpiA-pgm-eno.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG14085] |
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 52: | Line 52: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' transcription repression of the glycolytic ''[[gapA]]'' operon |
* '''Protein family:''' | * '''Protein family:''' | ||
| Line 63: | Line 63: | ||
* '''Domains:''' | * '''Domains:''' | ||
| + | ** DNA binding domain (H-T-H motif) (37–56) | ||
* '''Modification:''' | * '''Modification:''' | ||
| Line 68: | Line 69: | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:''' fructose 1.6-bisphosphate [http://www.ncbi.nlm.nih.gov/sites/entrez/12622823 PubMed] and dihydroxyacetone phosphate, glucose-6-phosphate and fructose-6-phosphate [http://www.ncbi.nlm.nih.gov/sites/entrez/18554327 PubMed] act as inducer and result in release of CggR from the DNA |
* '''Interactions:''' | * '''Interactions:''' | ||
| Line 76: | Line 77: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' complex with Fructose-6-Phosphate [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=65242 NCBI], effector binding domain [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=44226 NCBI] |
| − | * '''Swiss prot entry:''' | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/O32253] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU33950] |
| − | + | === Additional information=== | |
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
* '''Operon:''' | * '''Operon:''' | ||
| + | ** ''[[cggR]]-[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' | ||
| + | ** ''[[cggR]]-[[gapA]]'' | ||
| + | |||
| + | The primary mRNAs of the operon are highly unstable. The primary mRNA is subject to processing at the very end of the ''[[cggR]]'' open reading frame. This results in stable mature ''[[gapA]]'' and ''[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' mRNAs. The processing event requires the [[Rny]] protein. | ||
| + | |||
| + | * '''Sigma factor:''' [[SigA]] | ||
| − | * '''[[ | + | * '''Regulation:''' expression activated by glucose (77 fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed], [[CggR]] represses the operon in the absence of glycolytic sugars [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+12622823 PubMed] |
| − | * ''' | + | * '''Regulatory mechanism:''' repression |
| − | * ''' | + | * '''Database entries:''' [http://dbtbs.hgc.jp/COG/prom/cggR-gapA-pgk-tpiA-pgm-eno.html DBTBS] |
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 100: | Line 106: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' GP311 (in frame deletion), available in [[Stülke]] lab |
| − | * '''Expression vector:''' | + | * '''Expression vector:''' pGP705 (N-terminal His-tag, in [[pWH844]]), available in [[Stülke]] lab |
| − | + | ||
| − | * '''lacZ fusion:''' | + | * '''lacZ fusion:''' pGP504 (in [[pAC7]]), pGP509 (in [[pAC6]]), available in [[Stülke]] lab |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * ''' | + | * '''Antibody:''' available in [[Stülke]] lab |
| − | + | =Labs working on this gene/protein= | |
| − | + | [[Stephane Aymerich |Stephane Aymerich]], Microbiology and Molecular Genetics, INRA Paris-Grignon, France | |
=Your additional remarks= | =Your additional remarks= | ||
| Line 118: | Line 124: | ||
=References= | =References= | ||
| − | # | + | # Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in ''Bacillus subtilis'': regulation of the central metabolic pathways. ''Metab Eng.'' '''5:''' 133-149 [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] |
| − | # | + | # Commichau, F. M., Rothe, F. M., Herzberg, C., Wagner, E., Hellwig, D., Lehnik-Habrink, M., Hammer, E., Völker, U. & Stülke, J. Novel activities of glycolytic enzymes in Bacillus subtilis: Interactions with essential proteins involved in mRNA processing. subm. |
| − | # | + | # Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic pathways in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 47: 1709-1721. [http://www.ncbi.nlm.nih.gov/sites/entrez/12622823 PubMed] |
| + | # Doan et al. (2008) A phospho-sugar binding domain homologous to NagB enzymes regulates the activity of the central glycolytic genes repressor. Proteins 71:2038-2050. [http://www.ncbi.nlm.nih.gov/sites/entrez/18186488 PubMed] | ||
| + | # Fillinger, S., Boschi-Muller, S., Azza, S., Dervyn, E., Branlant, G., and Aymerich, S. (2000) Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J Biol Chem 275, 14031-14037. [http://www.ncbi.nlm.nih.gov/sites/entrez/10799476 PubMed] | ||
| + | # Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in ''Bacillus subtilis'': evidence for the presence of multiple levels of control of the ''gapA'' operon. Mol Microbiol 41, 409-422.[http://www.ncbi.nlm.nih.gov/sites/entrez/11489127 PubMed] | ||
| + | # Ludwig, H., Rebhan, N., Blencke, H.-M., Merzbacher, M. & Stülke, J. (2002). Control of the glycolytic ''gapA'' operon by the catabolite control protein A in ''Bacillus subtilis'': a novel mechanism of CcpA-mediated regulation. Mol Microbiol 45, 543-553.[http://www.ncbi.nlm.nih.gov/sites/entrez/12123463 PubMed] | ||
| + | # Meinken, C., Blencke, H. M., Ludwig, H., and Stülke, J. (2003) Expression of the glycolytic ''gapA'' operon in ''Bacillus subtilis'': differential synthesis of proteins encoded by the operon. Microbiology 149, 751-761. [http://www.ncbi.nlm.nih.gov/sites/entrez/12634343 PubMed] | ||
| + | # Rezacova et al. (2008) Crystal structures of the effector-binding domain of repressor Central glycolytic gene Regulator from Bacillus subtilis reveal ligand-induced structural changes upon binding of several glycolytic intermediates. Mol. Microbiol. 69:895-910. [http://www.ncbi.nlm.nih.gov/sites/entrez/18554327 PubMed] | ||
| + | # Zorilla et al. (2007) Fructose-1,6-bisphosphate acts both as an inducer and as a structural cofactor of the central glycolytic genes repressor (CggR). Biochemistry 46:14996-15008. [http://www.ncbi.nlm.nih.gov/sites/entrez/18052209 PubMed] | ||
| + | # Zorilla et al. (2007) Inducer-modulated cooperative binding of the tetrameric CggR repressor to operator DNA. Biophys. J. 92: 3215-3227. [http://www.ncbi.nlm.nih.gov/sites/entrez/17293407 PubMed] | ||
Revision as of 16:44, 15 April 2009
- Description: repressor of the glycolytic gapA operon
| Gene name | cggR |
| Synonyms | yvbQ |
| Essential | no |
| Product | central glycolytic genes regulator |
| Function | transcriptional regulator |
| MW, pI | 37,2 kDa,5.68 |
| Gene length, protein length | 1020 bp, 340 amino acids |
| Immediate neighbours | araE, gapA |
| Hier soll was neues rein | |
Genetic context 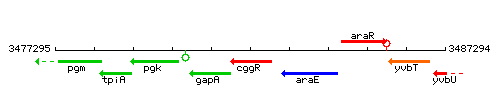
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates: 3481786 - 3482805
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription repression of the glycolytic gapA operon
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- DNA binding domain (H-T-H motif) (37–56)
- Modification:
- Cofactor(s):
- Effectors of protein activity: fructose 1.6-bisphosphate PubMed and dihydroxyacetone phosphate, glucose-6-phosphate and fructose-6-phosphate PubMed act as inducer and result in release of CggR from the DNA
- Interactions:
- Localization:
Database entries
- Swiss prot entry: [3]
- KEGG entry: [4]
Additional information
Expression and regulation
The primary mRNAs of the operon are highly unstable. The primary mRNA is subject to processing at the very end of the cggR open reading frame. This results in stable mature gapA and gapA-pgk-tpiA-pgm-eno mRNAs. The processing event requires the Rny protein.
- Sigma factor: SigA
- Regulation: expression activated by glucose (77 fold) PubMed, CggR represses the operon in the absence of glycolytic sugars PubMed
- Regulatory mechanism: repression
- Database entries: DBTBS
- Additional information:
Biological materials
- Mutant: GP311 (in frame deletion), available in Stülke lab
- GFP fusion:
- Antibody: available in Stülke lab
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Your additional remarks
References
- Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Eng. 5: 133-149 PubMed
- Commichau, F. M., Rothe, F. M., Herzberg, C., Wagner, E., Hellwig, D., Lehnik-Habrink, M., Hammer, E., Völker, U. & Stülke, J. Novel activities of glycolytic enzymes in Bacillus subtilis: Interactions with essential proteins involved in mRNA processing. subm.
- Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic pathways in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 47: 1709-1721. PubMed
- Doan et al. (2008) A phospho-sugar binding domain homologous to NagB enzymes regulates the activity of the central glycolytic genes repressor. Proteins 71:2038-2050. PubMed
- Fillinger, S., Boschi-Muller, S., Azza, S., Dervyn, E., Branlant, G., and Aymerich, S. (2000) Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J Biol Chem 275, 14031-14037. PubMed
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.PubMed
- Ludwig, H., Rebhan, N., Blencke, H.-M., Merzbacher, M. & Stülke, J. (2002). Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol Microbiol 45, 543-553.PubMed
- Meinken, C., Blencke, H. M., Ludwig, H., and Stülke, J. (2003) Expression of the glycolytic gapA operon in Bacillus subtilis: differential synthesis of proteins encoded by the operon. Microbiology 149, 751-761. PubMed
- Rezacova et al. (2008) Crystal structures of the effector-binding domain of repressor Central glycolytic gene Regulator from Bacillus subtilis reveal ligand-induced structural changes upon binding of several glycolytic intermediates. Mol. Microbiol. 69:895-910. PubMed
- Zorilla et al. (2007) Fructose-1,6-bisphosphate acts both as an inducer and as a structural cofactor of the central glycolytic genes repressor (CggR). Biochemistry 46:14996-15008. PubMed
- Zorilla et al. (2007) Inducer-modulated cooperative binding of the tetrameric CggR repressor to operator DNA. Biophys. J. 92: 3215-3227. PubMed