Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' phosphoglycerate mutase, glycolytic/ gluconeogenic enzyme<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''pgm'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''gpmI'' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || yes | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || 2,3-bisphosphoglycerate-independent <br/>phosphoglycerate mutase |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || enzyme in glycolysis/ gluconeogenesis | |style="background:#ABCDEF;" align="center"|'''Function''' || enzyme in glycolysis/ gluconeogenesis | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 56,1 kDa, 5.21 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1533 bp, 511 amino acids |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[tpi]]'', ''[[eno]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:pgm_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 29: | Line 29: | ||
__TOC__ | __TOC__ | ||
| − | <br/><br/> | + | <br/><br/><br/> |
| Line 36: | Line 36: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Coordinates:''' | + | * '''Coordinates:''' 3476911 - 3478443 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | + | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] |
=== Database entries === | === Database entries === | ||
| Line 46: | Line 46: | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/cggR-gapA-pgk-tpiA-pgm-eno.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/cggR-gapA-pgk-tpiA-pgm-eno.html] | ||
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10898] |
=== Additional information=== | === Additional information=== | ||
| Line 54: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' D- | + | * '''Catalyzed reaction/ biological activity:''' 2-phospho-D-glycerate = 3-phospho-D-glycerate |
| − | * '''Protein family:''' | + | * '''Protein family:''' BPG-independent phosphoglycerate mutase family |
* '''Paralogous protein(s):''' | * '''Paralogous protein(s):''' | ||
| Line 66: | Line 66: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' phosphorylation on Ser- | + | * '''Modification:''' phosphorylation on Ser-62 [http://www.ncbi.nlm.nih.gov/sites/entrez/17218307 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] |
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' 2 manganese ions per subunit |
| − | * '''Effectors of protein activity:''' inhibited by 2- | + | * '''Effectors of protein activity:''' inhibited by heavy-metal ions, 2,3-butanedione and sulfhydryl agents [http://www.ncbi.nlm.nih.gov/sites/entrez/33963 PubMed] |
| − | * '''Interactions:''' | + | * '''Interactions:''' Pgm-[[PfkA]] |
| − | * '''Localization:''' | + | * '''Localization:''' Cytoplasm (Homogeneous) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] |
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' complex with 2- | + | * '''Structure:''' ''Geobacillus stearothermophilus'', complex with 2-phosphoglycerate [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=16359 NCBI], ''Geobacillus stearothermophilus'', complex with 3-phosphoglycerate [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=15578 NCBI] |
| − | * '''Swiss prot entry:''' [http://www.expasy.ch/cgi-bin/sprot-search-ac? | + | * '''Swiss prot entry:''' [http://www.expasy.ch/cgi-bin/sprot-search-ac?P39773] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU33910] |
| − | * '''E.C. number:''' [http://www.expasy.org/enzyme/5. | + | * '''E.C. number:''' [http://www.expasy.org/enzyme/5.4.2.1 5.4.2.1] |
=== Additional information=== | === Additional information=== | ||
| + | is pH sensitive | ||
=Expression and regulation= | =Expression and regulation= | ||
| Line 96: | Line 97: | ||
* '''Sigma factor:''' [[SigA]] | * '''Sigma factor:''' [[SigA]] | ||
| − | * '''Regulation:''' expression activated by glucose ( | + | * '''Regulation:''' expression activated by glucose (7.3 fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] |
| − | + | ''[[cggR]]'': neg. regulated by [[CggR]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11489127 PubMed], induced by sugar | |
| − | + | ||
| + | ''[[pgk]]'': constitutive [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11489127 PubMed] | ||
* '''Regulatory mechanism:''' transcription repression by [[CggR]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11489127 PubMed] | * '''Regulatory mechanism:''' transcription repression by [[CggR]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11489127 PubMed] | ||
| Line 108: | Line 110: | ||
* '''Mutant:''' | * '''Mutant:''' | ||
| − | * '''Expression vector:''' | + | * '''Expression vector:''' pGP1101 (N-terminal His-tag, in [[pWH844]]), pGP396 (Pgm-S62A, N-terminal His-tag, in [[pWH844]]), pGP92 (N-terminal Strep-tag, for SPINE, expression in B. subtilis, in [[pGP380]]), available in [[Stülke]] lab |
| − | * '''lacZ fusion:''' | + | * '''lacZ fusion:''' |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| Line 119: | Line 121: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| + | |||
| + | [[Stülke|Jörg Stülke]], University of Göttingen, Germany [http://wwwuser.gwdg.de/~genmibio/stuelke.html Homepage] | ||
| + | |||
| + | [[Jedrzejas|Mark J. Jedrzejas]], Research Center Oakland, CA, USA [http://www.chori.org/Principal_Investigators/Jedrzejas_Mark_J/jedrzejas_research.html Homepage] | ||
=Your additional remarks= | =Your additional remarks= | ||
| Line 125: | Line 131: | ||
# Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in ''Bacillus subtilis'': regulation of the central metabolic pathways. ''Metab Eng.'' '''5:''' 133-149 [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | # Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in ''Bacillus subtilis'': regulation of the central metabolic pathways. ''Metab Eng.'' '''5:''' 133-149 [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | ||
| − | # | + | # Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in ''Bacillus subtilis''. ''Proteomics'' '''7:''' 3509-3526. [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] |
| + | # Meile et al. (2006) Systematic localisation of proteins fused to the green fluorescent protein in ''Bacillus subtilis'': identification of new proteins at the DNA replication factory ''Proteomics'' '''6:''' 2135-2146. [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] | ||
# Jannière, L., Canceill, D., Suski, C., Kanga, S., Dalmais, B., Lestini, R., Monnier, A. F., Chapuis, J., Bolotin, A., Titok, M., Le Chatelier, E., and Ehrlich, S. D. (2007) Genetic evidence for a link between glycolysis and DNA replication. PLoS ONE 2, e447. [http://www.ncbi.nlm.nih.gov/sites/entrez/17505547 PubMed] | # Jannière, L., Canceill, D., Suski, C., Kanga, S., Dalmais, B., Lestini, R., Monnier, A. F., Chapuis, J., Bolotin, A., Titok, M., Le Chatelier, E., and Ehrlich, S. D. (2007) Genetic evidence for a link between glycolysis and DNA replication. PLoS ONE 2, e447. [http://www.ncbi.nlm.nih.gov/sites/entrez/17505547 PubMed] | ||
| − | # Leyva-Vazquez, M. A., and Setlow, P. (1994) Cloning and nucleotide sequences of the genes encoding triose phosphate isomerase, phosphoglycerate mutase, and enolase from Bacillus subtilis. J Bacteriol 176: 3903-3910. [http://www.ncbi.nlm.nih.gov/sites/entrez/8021172 PubMed] | + | # Leyva-Vazquez, M. A., and Setlow, P. (1994) Cloning and nucleotide sequences of the genes encoding triose phosphate isomerase, phosphoglycerate mutase, and enolase from Bacillus subtilis. J Bacteriol 176: 3903-3910. [http://www.ncbi.nlm.nih.gov/sites/entrez/8021172 PubMed] |
| + | # Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M. & Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: Evidence for the presence of multiple levels of control of the gapA operon. Mol. Microbiol. 41: 409-422. [http://www.ncbi.nlm.nih.gov/sites/entrez/11489127 PubMed] | ||
| + | # Chandler et al. (1999) Structural studies on a 2,3-diphosphoglycerate independent phosphoglycerate mutase from Bacillus stearothermophilus. J. Struct. Biol. 126: 156-165. [http://www.ncbi.nlm.nih.gov/sites/entrez/10388626 PubMed] | ||
| + | # Jedrzejas et al. (2000) Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. EMBO J. 19: 1419-1431. [http://www.ncbi.nlm.nih.gov/sites/entrez/10747010 PubMed] | ||
| + | # Jedrzejas et al. (2000) Mechanism of catalysis of the cofactor-independent phosphoglycerate mutase from Bacillus stearothermophilus. Crystal structure of the complex with a 2-phosphoglycerate. J. Biol. Chem. 275: 23146-23153. [http://www.ncbi.nlm.nih.gov/sites/entrez/10764795 PubMed] | ||
| + | # Jedrzejas and Setlow (2001) Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: their mechanism of catalysis via a phosphoserine intermediate. Chem. Rev. 101: 607-618. [http://www.ncbi.nlm.nih.gov/sites/entrez/11712498 PubMed] | ||
| + | # Ridgen et al. (2003) Insights into the catalytic mechanism of cofactor-independent phosphoglycerate mutase from X-ray crystallography, simulated dynamics and molecular modeling. J. Mol. Biol. 328: 909-920. [http://www.ncbi.nlm.nih.gov/sites/entrez/12729763 PubMed] | ||
| + | # Nukui et al. (2007) Structure and molecular mechanism of Bacillus anthracis cofactor-independent phosphoglycerate mutase: a crucial enzyme for spores and growing cells of Bacillus species. Biophys. J. 92: 977-988. [http://www.ncbi.nlm.nih.gov/sites/entrez/17085493 PubMed] | ||
# Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium ''Bacillus subtilis''. Mol. Cell. Proteomics 6: 697-707 [http://www.ncbi.nlm.nih.gov/pubmed/17218307 PubMed] | # Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium ''Bacillus subtilis''. Mol. Cell. Proteomics 6: 697-707 [http://www.ncbi.nlm.nih.gov/pubmed/17218307 PubMed] | ||
Revision as of 16:44, 15 April 2009
- Description: phosphoglycerate mutase, glycolytic/ gluconeogenic enzyme
| Gene name | pgm |
| Synonyms | gpmI |
| Essential | yes |
| Product | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase |
| Function | enzyme in glycolysis/ gluconeogenesis |
| MW, pI | 56,1 kDa, 5.21 |
| Gene length, protein length | 1533 bp, 511 amino acids |
| Immediate neighbours | tpi, eno |
| Hier soll was neues rein | |
Genetic context 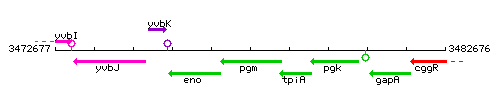
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates: 3476911 - 3478443
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2-phospho-D-glycerate = 3-phospho-D-glycerate
- Protein family: BPG-independent phosphoglycerate mutase family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s): 2 manganese ions per subunit
- Effectors of protein activity: inhibited by heavy-metal ions, 2,3-butanedione and sulfhydryl agents PubMed
- Interactions: Pgm-PfkA
- Localization: Cytoplasm (Homogeneous) PubMed
Database entries
- Structure: Geobacillus stearothermophilus, complex with 2-phosphoglycerate NCBI, Geobacillus stearothermophilus, complex with 3-phosphoglycerate NCBI
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: 5.4.2.1
Additional information
is pH sensitive
Expression and regulation
- Sigma factor: SigA
- Regulation: expression activated by glucose (7.3 fold) PubMed
cggR: neg. regulated by CggR PubMed, induced by sugar
- Additional information:
Biological materials
- Mutant:
- Expression vector: pGP1101 (N-terminal His-tag, in pWH844), pGP396 (Pgm-S62A, N-terminal His-tag, in pWH844), pGP92 (N-terminal Strep-tag, for SPINE, expression in B. subtilis, in pGP380), available in Stülke lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Mark J. Jedrzejas, Research Center Oakland, CA, USA Homepage
Your additional remarks
References
- Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Eng. 5: 133-149 PubMed
- Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 7: 3509-3526. PubMed
- Meile et al. (2006) Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory Proteomics 6: 2135-2146. PubMed
- Jannière, L., Canceill, D., Suski, C., Kanga, S., Dalmais, B., Lestini, R., Monnier, A. F., Chapuis, J., Bolotin, A., Titok, M., Le Chatelier, E., and Ehrlich, S. D. (2007) Genetic evidence for a link between glycolysis and DNA replication. PLoS ONE 2, e447. PubMed
- Leyva-Vazquez, M. A., and Setlow, P. (1994) Cloning and nucleotide sequences of the genes encoding triose phosphate isomerase, phosphoglycerate mutase, and enolase from Bacillus subtilis. J Bacteriol 176: 3903-3910. PubMed
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M. & Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: Evidence for the presence of multiple levels of control of the gapA operon. Mol. Microbiol. 41: 409-422. PubMed
- Chandler et al. (1999) Structural studies on a 2,3-diphosphoglycerate independent phosphoglycerate mutase from Bacillus stearothermophilus. J. Struct. Biol. 126: 156-165. PubMed
- Jedrzejas et al. (2000) Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. EMBO J. 19: 1419-1431. PubMed
- Jedrzejas et al. (2000) Mechanism of catalysis of the cofactor-independent phosphoglycerate mutase from Bacillus stearothermophilus. Crystal structure of the complex with a 2-phosphoglycerate. J. Biol. Chem. 275: 23146-23153. PubMed
- Jedrzejas and Setlow (2001) Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: their mechanism of catalysis via a phosphoserine intermediate. Chem. Rev. 101: 607-618. PubMed
- Ridgen et al. (2003) Insights into the catalytic mechanism of cofactor-independent phosphoglycerate mutase from X-ray crystallography, simulated dynamics and molecular modeling. J. Mol. Biol. 328: 909-920. PubMed
- Nukui et al. (2007) Structure and molecular mechanism of Bacillus anthracis cofactor-independent phosphoglycerate mutase: a crucial enzyme for spores and growing cells of Bacillus species. Biophys. J. 92: 977-988. PubMed
- Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6: 697-707 PubMed