Difference between revisions of "SinR"
(→Reviews) |
(→Original publications) |
||
| Line 187: | Line 187: | ||
<pubmed> 21095906 </pubmed> | <pubmed> 21095906 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed> 22893383 23378512 23430750 23475644 21856853 21815947 22329926,21326214,21708175 8955328, 15661000,8878039, 16923912,15104138,16430695,16430696,18047568,18430133,11751836,1906467,11751836,7635837,11751836, 19201793, 10547280, 15104138, 9799632 19788541 19898538 3125149 8932324 20351052 20923420 8422983 9685500 9158733 23646920 23660663 24256735 24347549 </pubmed> | + | <pubmed> 22893383 23378512 23430750 23475644 21856853 21815947 22329926,21326214,21708175 8955328, 15661000, 8878039, 24317403 16923912,15104138, 16430695,16430696,18047568, 18430133,11751836,1906467, 11751836, 7635837, 11751836, 19201793, 10547280, 15104138, 9799632 19788541 19898538 3125149 8932324 20351052 20923420 8422983 9685500 9158733 23646920 23660663 24256735 24347549 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:30, 30 November 2014
- Description: transcriptional regulator (Xre family) of post-exponential-phase responses genes
| Gene name | sinR |
| Synonyms | sin, flaD |
| Essential | no |
| Product | transcriptional regulator (Xre family) of post-exponential-phase responses genes |
| Function | control of biofilm formation |
| Gene expression levels in SubtiExpress: sinR | |
| Interactions involving this protein in SubtInteract: SinR | |
| Metabolic function and regulation of this protein in SubtiPathways: sinR | |
| MW, pI | 12 kDa, 7.177 |
| Gene length, protein length | 333 bp, 111 aa |
| Immediate neighbours | sinI, tasA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 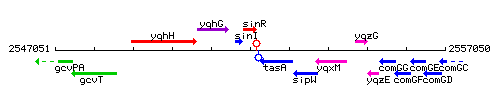
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, transition state regulators, biofilm formation
This gene is a member of the following regulons
AbrB regulon, ScoC regulon, Spo0A regulon
The SinR regulon
The gene
Basic information
- Locus tag: BSU24610
Phenotypes of a mutant
Database entries
- BsubCyc: BSU24610
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- transcription regulator of biofilm genes, acts as a true repressor of the tapA-sipW-tasA operon and as an anti-activator (prevents binding of the activator protein RemA) of the epsA-epsB-epsC-epsD-epsE-epsF-epsG-epsH-epsI-epsJ-epsK-epsL-epsM-epsN-epsO operon PubMed
- acts as co-repressor for SlrR PubMed
- Protein family:Xre family
- Paralogous protein(s): SlrR
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU24610
- Structure:
- UniProt: P06533
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- the mRNA is substantially stabilized upon depletion of RNase Y (the half-life of the mRNA increases from 3.5 to 13 min) PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 699 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 425 PubMed
Biological materials
- Mutant:
- GP923 (sinR::spec) PubMed, available in Jörg Stülke's lab
- GP736 (sinR::tetR) PubMed, available in Jörg Stülke's lab
- 1S97 (sinR::phleo), PubMed, available at BGSC
- GP1672 (sinR-tasA::cat) PubMed, available in Jörg Stülke's lab
- GP1663 (yghG-sinI-sinR-tasA), available in Jörg Stülke's lab
- Expression vector:
- N-terminal Strep-tag, for SPINE, expression in B. subtilis, in pGP380: pGP1083 , available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct: GP960 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Modelling of the SinI/SinR switch
Original publications