Difference between revisions of "RacX"
| Line 35: | Line 35: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 110: | Line 106: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=racX_3533419_3534102_-1 racX] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=racX_3533419_3534102_-1 racX] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigW]] {{PubMed|8491712,12207695}} | + | * '''[[Sigma factor]]:''' [[SigW]] {{PubMed|8491712,12207695}} |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 141: | Line 137: | ||
=References= | =References= | ||
| − | <pubmed>8491712,19063962,9987136, 20431016,7592498, </pubmed> | + | <pubmed>8491712,19063962,9987136, 20431016,7592498, 20817675</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 21:14, 18 June 2013
- Description: amino acid racemase, production of D-amino acids, control of biofilm formation
| Gene name | racX |
| Synonyms | |
| Essential | no |
| Product | amino acid racemase |
| Function | control of biofilm formation |
| Gene expression levels in SubtiExpress: racX | |
| MW, pI | 25 kDa, 5.396 |
| Gene length, protein length | 681 bp, 227 aa |
| Immediate neighbours | yveF, pbpE |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 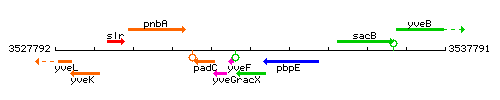
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed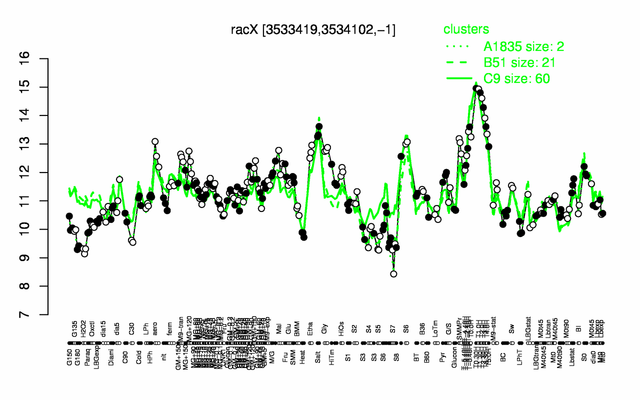
| |
Contents
Categories containing this gene/protein
biofilm formation, cell envelope stress proteins (controlled by SigM, V, W, X, Y)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU34430
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: aspartate/glutamate racemases family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P32960
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Ilana Kolodkin-Gal, Diego Romero, Shugeng Cao, Jon Clardy, Roberto Kolter, Richard Losick
D-amino acids trigger biofilm disassembly.
Science: 2010, 328(5978);627-9
[PubMed:20431016]
[WorldCat.org]
[DOI]
(I p)
María Mercedes Palomino, Carmen Sanchez-Rivas, Sandra M Ruzal
High salt stress in Bacillus subtilis: involvement of PBP4* as a peptidoglycan hydrolase.
Res Microbiol: 2009, 160(2);117-24
[PubMed:19063962]
[WorldCat.org]
[DOI]
(P p)
X Huang, A Gaballa, M Cao, J D Helmann
Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, sigma W.
Mol Microbiol: 1999, 31(1);361-71
[PubMed:9987136]
[WorldCat.org]
[DOI]
(P p)
M A Strauch
Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promote.
J Bacteriol: 1995, 177(23);6999-7002
[PubMed:7592498]
[WorldCat.org]
[DOI]
(P p)
D L Popham, P Setlow
Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpE operon, which codes for penicillin-binding protein 4* and an apparent amino acid racemase.
J Bacteriol: 1993, 175(10);2917-25
[PubMed:8491712]
[WorldCat.org]
[DOI]
(P p)