Difference between revisions of "GlpK"
Raphael2215 (talk | contribs) |
|||
| Line 13: | Line 13: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || glycerol utilization | |style="background:#ABCDEF;" align="center"|'''Function''' || glycerol utilization | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU09290 glpK] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/GlpK GlpK] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/GlpK GlpK] | ||
| Line 25: | Line 25: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glpF]]'', ''[[glpD]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glpF]]'', ''[[glpD]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU09290 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU09290 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU09290 Advanced_DNA] |
|- | |- | ||
|- | |- | ||
Revision as of 12:25, 13 May 2013
- Description: glycerol kinase
| Gene name | glpK |
| Synonyms | |
| Essential | no |
| Product | glycerol kinase |
| Function | glycerol utilization |
| Gene expression levels in SubtiExpress: glpK | |
| Interactions involving this protein in SubtInteract: GlpK | |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 54 kDa, 4.985 |
| Gene length, protein length | 1488 bp, 496 aa |
| Immediate neighbours | glpF, glpD |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 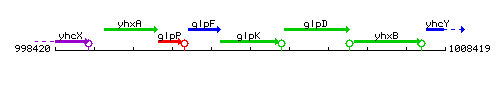
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed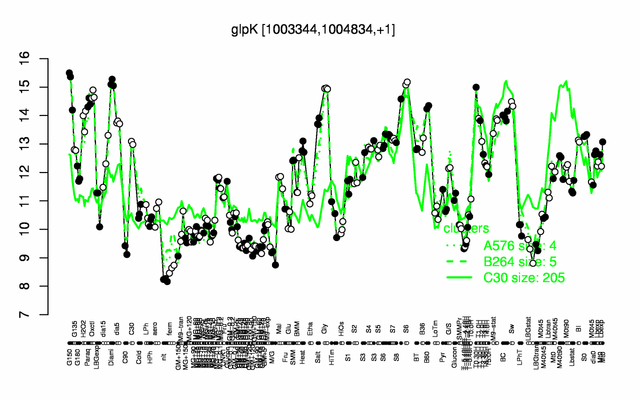
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources, phosphoproteins
This gene is a member of the following regulons
AbrB regulon, CcpA regulon, GlpP regulon
The gene
Basic information
- Locus tag: BSU09290
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + glycerol = ADP + sn-glycerol 3-phosphate (according to Swiss-Prot)
- Protein family: FGGY kinase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P18157
- KEGG entry: [3]
- E.C. number: 2.7.1.30
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Your additional remarks
References
Additional publications: PubMed
Joanne I Yeh, Regina Kettering, Ruth Saxl, Alexa Bourand, Emmanuelle Darbon, Nathalie Joly, Pierre Briozzo, Josef Deutscher
Structural characterizations of glycerol kinase: unraveling phosphorylation-induced long-range activation.
Biochemistry: 2009, 48(2);346-56
[PubMed:19102629]
[WorldCat.org]
[DOI]
(I p)
Emmanuelle Darbon, Pascale Servant, Sandrine Poncet, Josef Deutscher
Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression.
Mol Microbiol: 2002, 43(4);1039-52
[PubMed:11929549]
[WorldCat.org]
[DOI]
(P p)
V Charrier, E Buckley, D Parsonage, A Galinier, E Darbon, M Jaquinod, E Forest, J Deutscher, A Claiborne
Cloning and sequencing of two enterococcal glpK genes and regulation of the encoded glycerol kinases by phosphoenolpyruvate-dependent, phosphotransferase system-catalyzed phosphorylation of a single histidyl residue.
J Biol Chem: 1997, 272(22);14166-74
[PubMed:9162046]
[WorldCat.org]
[DOI]
(P p)
Christina Wehtje, Lena Beijer, Rune-Pär Nilsson, Blanka Rutberg
Mutations in the glycerol kinase gene restore the ability of a ptsGHI mutant of Bacillus subtilis to grow on glycerol.
Microbiology (Reading): 1995, 141 ( Pt 5);1193-1198
[PubMed:7773413]
[WorldCat.org]
[DOI]
(P p)
L Beijer, L Rutberg
Utilisation of glycerol and glycerol 3-phosphate is differently affected by the phosphotransferase system in Bacillus subtilis.
FEMS Microbiol Lett: 1992, 100(1-3);217-20
[PubMed:1335945]
[WorldCat.org]
[DOI]
(P p)