Difference between revisions of "Sandbox"
(→Expression and regulation) |
|||
| (7 intermediate revisions by one other user not shown) | |||
| Line 3: | Line 3: | ||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Gene name''' | + | |style="background:#ABCDEF;" align="center"|'''Gene name''' glaube ich oder nicht |
|''glmS'' | |''glmS'' | ||
|- | |- | ||
| Line 24: | Line 24: | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB11954]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB11954]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:quintos.gif]] |
| + | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |- | ||
| + | |colspan="2" | '''Genetic context''' <br/> [[Image:test.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 131: | Line 135: | ||
** subject to Clp-dependent proteolysis upon glucose starvation [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | ** subject to Clp-dependent proteolysis upon glucose starvation [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | ||
** A [[ncRNA]] is predicted between ''[[glmM]]'' and ''[[glmS]]'' {{PubMed|20525796}} | ** A [[ncRNA]] is predicted between ''[[glmM]]'' and ''[[glmS]]'' {{PubMed|20525796}} | ||
| − | ** number of protein molecules per cell (minimal medium with glucose and ammonium): | + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 2000 {{PubMed|24696501}} |
| − | ** number of protein molecules per cell (complex medium with amino acids, without glucose): | + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 4000 {{PubMed|24696501}} |
=Biological materials = | =Biological materials = | ||
Latest revision as of 13:22, 29 July 2014
- Description: glutamine-fructose-6-phosphate transaminase
| Gene name glaube ich oder nicht | glmS |
| Synonyms | gcaA, ybxD |
| Essential | yes PubMed |
| Product | glutamine-fructose-6-phosphate transaminase |
| Function | cell wall synthesis |
| Metabolic function and regulation of this protein in SubtiPathways: sandbox | |
| MW, pI | 65 kDa, 4.796 |
| Gene length, protein length | 1800 bp, 600 aa |
| Immediate neighbours | glmM, ybbU |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
| Genetic context File:Quintos.gif This image was kindly provided by SubtiList
| |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed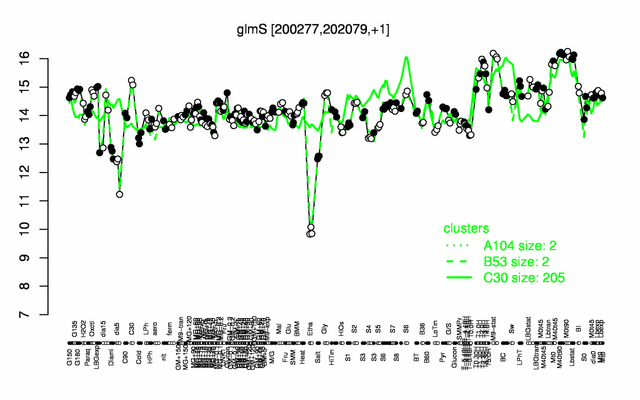
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01780
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: [HELLO BSU00100]
- BsubCyc: "
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-glutamine + D-fructose 6-phosphate = L-glutamate + D-glucosamine 6-phosphate (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: [HELLO BSU00100]
- BsubCyc: BSU00240
- Structure:
- UniProt: P39754
- KEGG entry: [2]
- E.C. number: 2.6.1.16
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation:
- Regulatory mechanism: glmS ribozyme: glucosamine 6-phosphate binds the leader mRNA, and a riboswitch with ribozyme activity cleaves off the glmS section from the mRNA, resulting in stopp of transcript elongation
- Additional information:
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
- A ncRNA is predicted between glmM and glmS PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 2000 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 4000 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Wade Winkler, University of Texas, USA, Homepage
Your additional remarks
References
Reviews
The glmS Ribozyme
Other Original Publications
Additional publications: PubMed