Mfd
- Description: transcription-repair coupling factor, eliminates genetic damage from transcriptionally active genes during sporulation
| Gene name | mfd |
| Synonyms | |
| Essential | no |
| Product | transcription-repair coupling factor |
| Function | promotes strand-specific DNA repair by displacing
RNA polymerase stalled at a nucleotide lesion and directing the (A)BC excinuclease to the RNA damage site |
| Gene expression levels in SubtiExpress: mfd
RNA polymerase stalled at a nucleotide lesion and directing the (A)BC excinuclease to the RNA damage site | |
| Interactions involving this protein in SubtInteract: Mfd | |
| MW, pI | 133 kDa, 5.367 |
| Gene length, protein length | 3531 bp, 1177 aa |
| Immediate neighbours | fin, spoVT |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 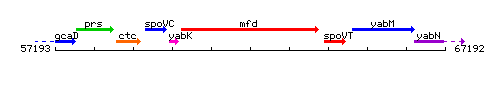
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
DNA repair/ recombination, transcription
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00550
Phenotypes of a mutant
- in an mfd knock-out, the cell's ability to accumulate adaptive mutations in stationary phase is depressed. PubMed
- increased UV-induced mutagenesis via PolY1/ PolY2-mediated translesion synthesis PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- promotes strand-specific DNA repair by displacing RNA polymerase stalled at a nucleotide lesion and directing the (A)BC excinuclease to the RNA damage site
- is required for roadblock transcription repression by transcription factors with binding sites downstream of the promoter (as for CcpA PubMed and CodY PubMed)
- Protein family:
- Paralogous protein(s): RecG
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P37474
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
- expressed throughout growth and sporulation, during sporulation expressed both in the forespore and the mother cell PubMed
Biological materials
- Mutant: GP1167 (del ermC), available in Stülke lab
- Expression vector:
- lacZ fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Justin S Lenhart, Jeremy W Schroeder, Brian W Walsh, Lyle A Simmons
DNA repair and genome maintenance in Bacillus subtilis.
Microbiol Mol Biol Rev: 2012, 76(3);530-64
[PubMed:22933559]
[WorldCat.org]
[DOI]
(I p)
Ann Ganesan, Graciela Spivak, Philip C Hanawalt
Transcription-coupled DNA repair in prokaryotes.
Prog Mol Biol Transl Sci: 2012, 110;25-40
[PubMed:22749141]
[WorldCat.org]
[DOI]
(I p)
Eduardo A Robleto, Holly A Martin, Mario Pedraza-Reyes
Mfd and transcriptional derepression cause genetic diversity in Bacillus subtilis.
Front Biosci (Elite Ed): 2012, 4(4);1246-54
[PubMed:22201950]
[WorldCat.org]
[DOI]
(I e)
Philip C Hanawalt, Graciela Spivak
Transcription-coupled DNA repair: two decades of progress and surprises.
Nat Rev Mol Cell Biol: 2008, 9(12);958-70
[PubMed:19023283]
[WorldCat.org]
[DOI]
(I p)
Rodrigo S Galhardo, P J Hastings, Susan M Rosenberg
Mutation as a stress response and the regulation of evolvability.
Crit Rev Biochem Mol Biol: 2007, 42(5);399-435
[PubMed:17917874]
[WorldCat.org]
[DOI]
(P p)
Alexandra M Deaconescu, Nigel Savery, Seth A Darst
The bacterial transcription repair coupling factor.
Curr Opin Struct Biol: 2007, 17(1);96-102
[PubMed:17239578]
[WorldCat.org]
[DOI]
(P p)
James J Truglio, Deborah L Croteau, Bennett Van Houten, Caroline Kisker
Prokaryotic nucleotide excision repair: the UvrABC system.
Chem Rev: 2006, 106(2);233-52
[PubMed:16464004]
[WorldCat.org]
[DOI]
(P p)
Sergei Borukhov, Jookyung Lee, Oleg Laptenko
Bacterial transcription elongation factors: new insights into molecular mechanism of action.
Mol Microbiol: 2005, 55(5);1315-24
[PubMed:15720542]
[WorldCat.org]
[DOI]
(P p)
Jeffrey Roberts, Joo-Seop Park
Mfd, the bacterial transcription repair coupling factor: translocation, repair and termination.
Curr Opin Microbiol: 2004, 7(2);120-5
[PubMed:15063847]
[WorldCat.org]
[DOI]
(P p)
E C Friedberg
Relationships between DNA repair and transcription.
Annu Rev Biochem: 1996, 65;15-42
[PubMed:8811173]
[WorldCat.org]
[DOI]
(P p)
C P Selby, A Sancar
Mechanisms of transcription-repair coupling and mutation frequency decline.
Microbiol Rev: 1994, 58(3);317-29
[PubMed:7968917]
[WorldCat.org]
[DOI]
(P p)
Original publications
Fernando H Ramírez-Guadiana, Rocío Del Carmen Barajas-Ornelas, Víctor M Ayala-García, Ronald E Yasbin, Eduardo Robleto, Mario Pedraza-Reyes
Transcriptional coupling of DNA repair in sporulating Bacillus subtilis cells.
Mol Microbiol: 2013, 90(5);1088-99
[PubMed:24118570]
[WorldCat.org]
[DOI]
(I p)
Kévin Howan, Abigail J Smith, Lars F Westblade, Nicolas Joly, Wilfried Grange, Sylvain Zorman, Seth A Darst, Nigel J Savery, Terence R Strick
Initiation of transcription-coupled repair characterized at single-molecule resolution.
Nature: 2012, 490(7420);431-4
[PubMed:22960746]
[WorldCat.org]
[DOI]
(I p)
Holly Anne Martin, Mario Pedraza-Reyes, Ronald E Yasbin, Eduardo A Robleto
Transcriptional de-repression and Mfd are mutagenic in stressed Bacillus subtilis cells.
J Mol Microbiol Biotechnol: 2011, 21(1-2);45-58
[PubMed:22248542]
[WorldCat.org]
[DOI]
(I p)
Katrin Gunka, Stefan Tholen, Jan Gerwig, Christina Herzberg, Jörg Stülke, Fabian M Commichau
A high-frequency mutation in Bacillus subtilis: requirements for the decryptification of the gudB glutamate dehydrogenase gene.
J Bacteriol: 2012, 194(5);1036-44
[PubMed:22178973]
[WorldCat.org]
[DOI]
(I p)
Olivier Delumeau, François Lecointe, Jan Muntel, Alain Guillot, Eric Guédon, Véronique Monnet, Michael Hecker, Dörte Becher, Patrice Polard, Philippe Noirot
The dynamic protein partnership of RNA polymerase in Bacillus subtilis.
Proteomics: 2011, 11(15);2992-3001
[PubMed:21710567]
[WorldCat.org]
[DOI]
(I p)
Boris R Belitsky, Abraham L Sonenshein
Roadblock repression of transcription by Bacillus subtilis CodY.
J Mol Biol: 2011, 411(4);729-43
[PubMed:21699902]
[WorldCat.org]
[DOI]
(I p)
Lars F Westblade, Elizabeth A Campbell, Chirangini Pukhrambam, Julio C Padovan, Bryce E Nickels, Valerie Lamour, Seth A Darst
Structural basis for the bacterial transcription-repair coupling factor/RNA polymerase interaction.
Nucleic Acids Res: 2010, 38(22);8357-69
[PubMed:20702425]
[WorldCat.org]
[DOI]
(I p)
Christine Pybus, Mario Pedraza-Reyes, Christian A Ross, Holly Martin, Katherine Ona, Ronald E Yasbin, Eduardo Robleto
Transcription-associated mutation in Bacillus subtilis cells under stress.
J Bacteriol: 2010, 192(13);3321-8
[PubMed:20435731]
[WorldCat.org]
[DOI]
(I p)
Christian Ross, Christine Pybus, Mario Pedraza-Reyes, Huang-Mo Sung, Ronald E Yasbin, Eduardo Robleto
Novel role of mfd: effects on stationary-phase mutagenesis in Bacillus subtilis.
J Bacteriol: 2006, 188(21);7512-20
[PubMed:16950921]
[WorldCat.org]
[DOI]
(P p)
Alexandra M Deaconescu, Anna L Chambers, Abigail J Smith, Bryce E Nickels, Ann Hochschild, Nigel J Savery, Seth A Darst
Structural basis for bacterial transcription-coupled DNA repair.
Cell: 2006, 124(3);507-20
[PubMed:16469698]
[WorldCat.org]
[DOI]
(P p)
J M Zalieckas, L V Wray, A E Ferson, S H Fisher
Transcription-repair coupling factor is involved in carbon catabolite repression of the Bacillus subtilis hut and gnt operons.
Mol Microbiol: 1998, 27(5);1031-8
[PubMed:9535092]
[WorldCat.org]
[DOI]
(P p)
S Ayora, F Rojo, N Ogasawara, S Nakai, J C Alonso
The Mfd protein of Bacillus subtilis 168 is involved in both transcription-coupled DNA repair and DNA recombination.
J Mol Biol: 1996, 256(2);301-18
[PubMed:8594198]
[WorldCat.org]
[DOI]
(P p)
V D Filippov, E E Zagoruiko
Study of MFD in Bacillus subtilis.
Mutat Res: 1978, 52(1);49-56
[PubMed:104170]
[WorldCat.org]
[DOI]
(P p)