Difference between revisions of "SpoIVFB"
| Line 29: | Line 29: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=spoIVFB_2855973_2856839_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:spoIVFB_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=spoIVFB_2855973_2856839_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:spoIVFB_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU27970]] |
|- | |- | ||
|} | |} | ||
Revision as of 14:09, 16 May 2013
- Description: intramembrane metalloprotease, processing of pro-sigma-K to active SigK
| Gene name | spoIVFB |
| Synonyms | |
| Essential | no |
| Product | intramembrane metalloprotease |
| Function | processing of pro-sigma-K to active SigK |
| Gene expression levels in SubtiExpress: spoIVFB | |
| Interactions involving this protein in SubtInteract: SpoIVFB | |
| MW, pI | 33 kDa, 8.483 |
| Gene length, protein length | 864 bp, 288 aa |
| Immediate neighbours | rplU, spoIVFA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 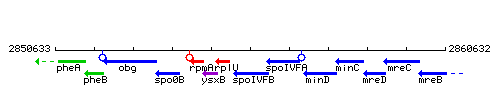
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed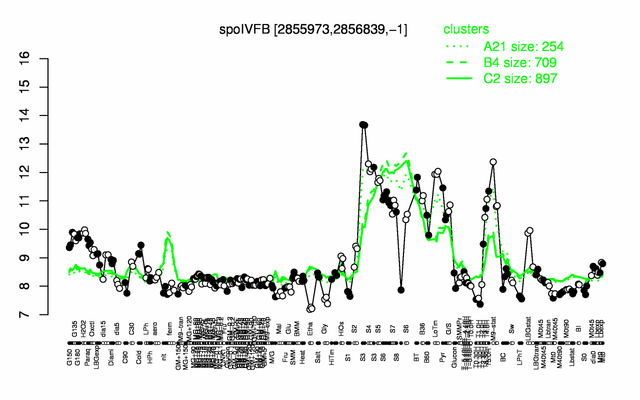
| |
Contents
Categories containing this gene/protein
sigma factors and their control, proteolysis, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27970
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family: peptidase M50B family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- C-terminal cystathionine-beta-synthase (CBS) domain, this domain binds ATP PubMed
- Modification:
- Cofactor(s):
- Effectors of protein activity: ATP regulates substrate access to the active site and renders cleavage sensitive to the cellular energy level PubMed
- Localization:
- cell membrane
Database entries
- Structure:
- UniProt: P26937
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Noël Molière, Kürşad Turgay
General and regulatory proteolysis in Bacillus subtilis.
Subcell Biochem: 2013, 66;73-103
[PubMed:23479438]
[WorldCat.org]
[DOI]
(P p)
Gu Chen, Xu Zhang
New insights into S2P signaling cascades: regulation, variation, and conservation.
Protein Sci: 2010, 19(11);2015-30
[PubMed:20836086]
[WorldCat.org]
[DOI]
(I p)
Original Publications
Ruanbao Zhou, Kangming Chen, Xianling Xiang, Liping Gu, Lee Kroos
Features of Pro-σK important for cleavage by SpoIVFB, an intramembrane metalloprotease.
J Bacteriol: 2013, 195(12);2793-806
[PubMed:23585539]
[WorldCat.org]
[DOI]
(I p)
Ruanbao Zhou, Christina Cusumano, Dexin Sui, R Michael Garavito, Lee Kroos
Intramembrane proteolytic cleavage of a membrane-tethered transcription factor by a metalloprotease depends on ATP.
Proc Natl Acad Sci U S A: 2009, 106(38);16174-9
[PubMed:19805276]
[WorldCat.org]
[DOI]
(I p)
Nathalie Campo, David Z Rudner
A branched pathway governing the activation of a developmental transcription factor by regulated intramembrane proteolysis.
Mol Cell: 2006, 23(1);25-35
[PubMed:16818230]
[WorldCat.org]
[DOI]
(P p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
Patrick Eichenberger, Masaya Fujita, Shane T Jensen, Erin M Conlon, David Z Rudner, Stephanie T Wang, Caitlin Ferguson, Koki Haga, Tsutomu Sato, Jun S Liu, Richard Losick
The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis.
PLoS Biol: 2004, 2(10);e328
[PubMed:15383836]
[WorldCat.org]
[DOI]
(I p)
Tran C Dong, Simon M Cutting
SpoIVB-mediated cleavage of SpoIVFA could provide the intercellular signal to activate processing of Pro-sigmaK in Bacillus subtilis.
Mol Microbiol: 2003, 49(5);1425-34
[PubMed:12940997]
[WorldCat.org]
[DOI]
(P p)
David Z Rudner, Qi Pan, Richard M Losick
Evidence that subcellular localization of a bacterial membrane protein is achieved by diffusion and capture.
Proc Natl Acad Sci U S A: 2002, 99(13);8701-6
[PubMed:12060714]
[WorldCat.org]
[DOI]
(P p)
David Z Rudner, Richard Losick
A sporulation membrane protein tethers the pro-sigmaK processing enzyme to its inhibitor and dictates its subcellular localization.
Genes Dev: 2002, 16(8);1007-18
[PubMed:11959848]
[WorldCat.org]
[DOI]
(P p)
D Z Rudner, P Fawcett, R Losick
A family of membrane-embedded metalloproteases involved in regulated proteolysis of membrane-associated transcription factors.
Proc Natl Acad Sci U S A: 1999, 96(26);14765-70
[PubMed:10611287]
[WorldCat.org]
[DOI]
(P p)
O Resnekov, R Losick
Negative regulation of the proteolytic activation of a developmental transcription factor in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1998, 95(6);3162-7
[PubMed:9501233]
[WorldCat.org]
[DOI]
(P p)
O Resnekov, S Alper, R Losick
Subcellular localization of proteins governing the proteolytic activation of a developmental transcription factor in Bacillus subtilis.
Genes Cells: 1996, 1(6);529-42
[PubMed:9078383]
[WorldCat.org]
[DOI]
(P p)
E Ricca, S Cutting, R Losick
Characterization of bofA, a gene involved in intercompartmental regulation of pro-sigma K processing during sporulation in Bacillus subtilis.
J Bacteriol: 1992, 174(10);3177-84
[PubMed:1577688]
[WorldCat.org]
[DOI]
(P p)
S Cutting, S Roels, R Losick
Sporulation operon spoIVF and the characterization of mutations that uncouple mother-cell from forespore gene expression in Bacillus subtilis.
J Mol Biol: 1991, 221(4);1237-56
[PubMed:1942049]
[WorldCat.org]
[DOI]
(P p)