Difference between revisions of "PfkA"
(→Biological materials) |
|||
| Line 111: | Line 111: | ||
* '''Mutant:''' | * '''Mutant:''' | ||
| − | * '''Expression vector:''' | + | * '''Expression vector:''' |
| − | + | ** for expression/ purification from ''B. subtilis'' with N-terminal Strep-tag, for [[SPINE]], in [[pGP380]]: pGP87, available in [[Stülke]] lab | |
| + | ** for expression/ purification from ''E. coli'' with N-terminal His-tag, in [[pWH844]]: pGP393, available in [[Stülke]] lab | ||
| + | |||
* '''lacZ fusion:''' pGP511 (in [[pAC6]]), available in [[Stülke]] lab | * '''lacZ fusion:''' pGP511 (in [[pAC6]]), available in [[Stülke]] lab | ||
Revision as of 18:11, 1 September 2009
- Description: phosphofructokinase, glycolytic enzyme
| Gene name | pfkA |
| Synonyms | pfk |
| Essential | yes |
| Product | 6-phosphofructokinase |
| Function | catabolic enzyme in glycolysis |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Sugar catabolism | |
| MW, pI | 34,1 kDa, 6.14 |
| Gene length, protein length | 957 bp, 319 amino acids |
| Immediate neighbours | accA, pyk |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 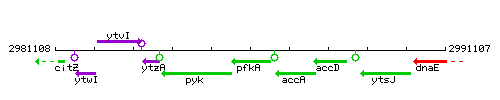
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU29190
Phenotypes of a mutant
- Essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + D-fructose 6-phosphate = ADP + D-fructose 1,6-bisphosphate (according to Swiss-Prot)
- Protein family: phosphofructokinase family (according to Swiss-Prot) phosphofructokinase family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Allosteric Regulation (Reversible) PubMed
- Domains:
- 3 x nucleotide binding domain (ATP) (21–25), (154–158), (171–187)
- Modification:
- Cofactor(s): Mg2+
- Effectors of protein activity:
- Inhibited by citrate, PEP (Hill Coefficient 3) and Ca2+ (competes with Mg2+) in B. licheniformes PubMed.
- Inhibited by ATP (competitively) and f6p (non-competitively) in G. stearothermophillus PubMed
- Activated by GDP and ADP in lower concentrations (1mM); above that inhibition, competing with the ATP for the binding site (in G. stearothermophillus) PubMed
- Activated by NH4+ PubMed
- Localization: cytoplasm (according to Swiss-Prot), Cytoplasm (Homogeneous) PubMed
Database entries
- Structure: 1MTO (mutant, complex with fructose-6-phosphate, Geobacillus stearothermophilus), 4PFK (Geobacillus stearothermophilus), Geobacillus stearothermophilus NCBI, Mutant form, complex with fructose-6-phosphate Geobacillus stearothermophilus NCBI
- UniProt: O34529
- KEGG entry: [3]
- E.C. number: 2.7.1.11
Additional information
Expression and regulation
- Sigma factor:
- Regulation: twofold induced by glucose PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References