Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' HPr kinase/ phosphorylase<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''hprK'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''ptsK, yvoB '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || HPr kinase/ phosphorylase |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || carbon catabolite repression, |
| + | phosphorylation of HPr and Crh proteins at Ser46 | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 34 kDa, 4.906 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 930 bp, 310 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[lgt]]'', ''[[nagA]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:hprK_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 38: | Line 39: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | |||
| + | no carbon catabolite repression | ||
=== Database entries === | === Database entries === | ||
| Line 43: | Line 46: | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG14125] |
=== Additional information=== | === Additional information=== | ||
| Line 70: | Line 73: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''Interactions:''' HprK-[[PtsH| HPr]], HprK-[[Crh]] [http://www.ncbi.nlm.nih.gov/sites/entrez/12009882 PubMed] |
* '''Localization:''' | * '''Localization:''' | ||
| Line 80: | Line 83: | ||
* '''Swiss prot entry:''' | * '''Swiss prot entry:''' | ||
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU35000] |
* '''E.C. number:''' | * '''E.C. number:''' | ||
| Line 90: | Line 93: | ||
* '''Operon:''' | * '''Operon:''' | ||
| − | * ''' | + | * '''Sigma factor:''' |
| − | * '''Regulation:''' | + | * '''Regulation:''' |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' |
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 113: | Line 116: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| + | |||
| + | [[Josef Deutscher]], Paris-Grignon, France | ||
| + | |||
| + | [[Stülke|Jörg Stülke]], University of Göttingen, Germany [http://wwwuser.gwdg.de/~genmibio/stuelke.html Homepage] | ||
| + | |||
| + | [[Wolfgang Hillen]], Erlangen University, Germany [http://www.biologie.uni-erlangen.de/mibi/index2.html Homepage] | ||
| + | |||
| + | [[Anne Galinier]], University of Marseille, France | ||
=Your additional remarks= | =Your additional remarks= | ||
| Line 118: | Line 129: | ||
=References= | =References= | ||
| − | # | + | '''review''' |
| − | # | + | # Nessler S, Fieulaine S, Poncet S, Galinier A, Deutscher J, Janin J (2003) HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in Gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate. J Bacteriol 185:4003-4010. [http://www.ncbi.nlm.nih.gov/sites/entrez/12837773 PubMed] |
| + | |||
| + | '''general/ physiology''' | ||
| + | # Reizer, J., Hoischen, C., Titgemeyer, F., Rivolta, C., Rabus, R., Stülke, J., Karamata, D., Saier, M. H., Jr., & Hillen, W. (1998) A novel bacterial protein kinase that controls carbon catabolite repression. Mol. Microbiol. 27: 1157-1169. [http://www.ncbi.nlm.nih.gov/sites/entrez/9570401 PubMed] | ||
| + | # Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer MC, Deutscher J, Haiech J (1998) New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA 95:1823-1828. [http://www.ncbi.nlm.nih.gov/sites/entrez/9465101 PubMed] | ||
| + | # Ludwig, H., Rebhan, N., Blencke, H.-M., Merzbacher, M. & Stülke, J. (2002) Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45: 543-553. [http://www.ncbi.nlm.nih.gov/sites/entrez/12123463 PubMed] | ||
| + | # Singh, K. D., Schmalisch, M. H., Stülke, J. & Görke, B. (2008) Carbon catabolite repression in Bacillus subtilis: A quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 190: 7275-7284. [http://www.ncbi.nlm.nih.gov/sites/entrez/18757537 PubMed] | ||
| + | |||
| + | '''enzymatic properties, mutation analysis''' | ||
| + | # Mijakovic I, Poncet S, Galiner A, Monedero V, Fieulaine S, Janin J, Nessler S, Marquez JA, Scheffzek K, Hasenbein S, Hengstenberg W, Deutscher J: Pyrophosphate-producing protein dephosphorylation by HPr kinase/ phosphorylase: a relic of early life? Proc Natl Acad Sci USA 2002, 99:13442-13447. [http://www.ncbi.nlm.nih.gov/sites/entrez/12359880 PubMed] | ||
| + | # Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonet V, Martin-Verstraete I, Nessler S, Deutscher J (2001) Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J 20:3928-3937. [http://www.ncbi.nlm.nih.gov/sites/entrez/11483496 PubMed] | ||
| + | # Hanson K. G., Steinhauer, K., Reizer, J., Hillen, W. & Stülke, J. (2002) HPr kinase/phosphatase of Bacillus subtilis: Expression of the gene and effects of mutations on enzyme activity, growth, and carbon catabolite repression. Microbiology 148: 1805-1811. [http://www.ncbi.nlm.nih.gov/sites/entrez/12055300 PubMed] | ||
| + | # Jault J M, Fieulaine S, Nessler S, Gonzalo P, Di Pietro A, Deutscher J, Galinier A (2000) The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J Biol Chem 275:1773-1780. [http://www.ncbi.nlm.nih.gov/sites/entrez/10636874 PubMed] | ||
| + | # Ramström H, Sanglier S, Leize-Wagner E, Philippe C, van Dorsselaer A, Haiech J (2003) Properties and regulation of the bifunctional enzyme HPr kinase/phosphatase in Bacillus subtilis. J Biol Chem 278:1174-1185. [http://www.ncbi.nlm.nih.gov/sites/entrez/12411438 PubMed] | ||
| + | # Galinier, A., Lavergne, J.-P., Geourjon, C., Fieulaine, S., Nessler, S. & Jault, J.-M. (2002) A new family of phosphotransferases with a P-loop motif. J. Biol. Chem. 277, 11362-11367. [http://www.ncbi.nlm.nih.gov/sites/entrez/11796714 PubMed] | ||
| + | # Lavergne, J.-P., Jault, J.-M. & Galinier, A. (2002) Insights into the functioning of ''Bacillus subtilis'' HPr kinase/phosphatase: affinity for its protein substrates and role of cations and phosphate. Biochemistry 41, 6218-6225. [http://www.ncbi.nlm.nih.gov/sites/entrez/12009882 PubMed] | ||
| + | # Pompeo, F., Granet, Y., Lavergne, J.-P., Grangeasse, C., Nessler, S., Jault, J.-M. & Galinier, A. (2003) Regulation and mutational analysis of the HPr kinase/phosphorylase from ''Bacillus subtilis''. Biochemistry 42, 6762-6771. [http://www.ncbi.nlm.nih.gov/sites/entrez/12779331 PubMed] | ||
| + | |||
| + | '''structure analysis''' | ||
| + | # Márquez, J. A., Hasenbein, S., Koch, B., Fieulaine, S., Nessler, S., Russell, R. B., Hengstenberg, W. & Scheffzek, K. (2002) Structure of the full-length HPr kinase/phosphatase from ''Staphylococcus xylosus'' at 1.95 Å resolution: mimicking the product/substrate of the phosphotransfer reactions. Proc. Natl. Acad. Sci. USA 99, 3458-3463. [http://www.ncbi.nlm.nih.gov/sites/entrez/11904409 PubMed] | ||
| + | # Allen, G.S., Steinhauer, K., Hillen, W., Stülke, J. & Brennan, R.G. (2003) Crystal structure of HPr kinase/phosphatase from ''Mycoplasma pneumoniae''. J. Mol. Biol. 326, 1203-1217. [http://www.ncbi.nlm.nih.gov/sites/entrez/12589763 PubMed] | ||
| + | # Fieulaine, S., Morera, S., Poncet, S., Mijakovic, I., Galinier, A., Janin, J., Deutscher, J., and Nessler, S. (2002) X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr. Proc Natl Acad Sci U S A 99: 13437-13441. [http://www.ncbi.nlm.nih.gov/sites/entrez/12359875 PubMed] | ||
| + | |||
| + | '''HprK as target for antimicrobial compounds''' | ||
| + | # Ramström, H. et al. (2004) Heterocylic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/ phosphatase from ''Bacillus subtilis''. J. Med. Chem. 47, 2264-2275. [http://www.ncbi.nlm.nih.gov/sites/entrez/15084125 PubMed] | ||
| + | |||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 16:36, 15 April 2009
- Description: HPr kinase/ phosphorylase
| Gene name | hprK |
| Synonyms | ptsK, yvoB |
| Essential | no |
| Product | HPr kinase/ phosphorylase |
| Function | carbon catabolite repression,
phosphorylation of HPr and Crh proteins at Ser46 |
| MW, pI | 34 kDa, 4.906 |
| Gene length, protein length | 930 bp, 310 aa |
| Immediate neighbours | lgt, nagA |
| Hier soll was neues rein | |
Genetic context 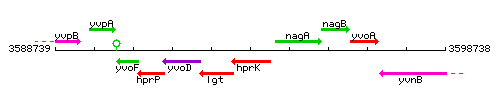
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
no carbon catabolite repression
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Wolfgang Hillen, Erlangen University, Germany Homepage
Anne Galinier, University of Marseille, France
Your additional remarks
References
review
- Nessler S, Fieulaine S, Poncet S, Galinier A, Deutscher J, Janin J (2003) HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in Gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate. J Bacteriol 185:4003-4010. PubMed
general/ physiology
- Reizer, J., Hoischen, C., Titgemeyer, F., Rivolta, C., Rabus, R., Stülke, J., Karamata, D., Saier, M. H., Jr., & Hillen, W. (1998) A novel bacterial protein kinase that controls carbon catabolite repression. Mol. Microbiol. 27: 1157-1169. PubMed
- Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer MC, Deutscher J, Haiech J (1998) New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA 95:1823-1828. PubMed
- Ludwig, H., Rebhan, N., Blencke, H.-M., Merzbacher, M. & Stülke, J. (2002) Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45: 543-553. PubMed
- Singh, K. D., Schmalisch, M. H., Stülke, J. & Görke, B. (2008) Carbon catabolite repression in Bacillus subtilis: A quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 190: 7275-7284. PubMed
enzymatic properties, mutation analysis
- Mijakovic I, Poncet S, Galiner A, Monedero V, Fieulaine S, Janin J, Nessler S, Marquez JA, Scheffzek K, Hasenbein S, Hengstenberg W, Deutscher J: Pyrophosphate-producing protein dephosphorylation by HPr kinase/ phosphorylase: a relic of early life? Proc Natl Acad Sci USA 2002, 99:13442-13447. PubMed
- Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonet V, Martin-Verstraete I, Nessler S, Deutscher J (2001) Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J 20:3928-3937. PubMed
- Hanson K. G., Steinhauer, K., Reizer, J., Hillen, W. & Stülke, J. (2002) HPr kinase/phosphatase of Bacillus subtilis: Expression of the gene and effects of mutations on enzyme activity, growth, and carbon catabolite repression. Microbiology 148: 1805-1811. PubMed
- Jault J M, Fieulaine S, Nessler S, Gonzalo P, Di Pietro A, Deutscher J, Galinier A (2000) The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J Biol Chem 275:1773-1780. PubMed
- Ramström H, Sanglier S, Leize-Wagner E, Philippe C, van Dorsselaer A, Haiech J (2003) Properties and regulation of the bifunctional enzyme HPr kinase/phosphatase in Bacillus subtilis. J Biol Chem 278:1174-1185. PubMed
- Galinier, A., Lavergne, J.-P., Geourjon, C., Fieulaine, S., Nessler, S. & Jault, J.-M. (2002) A new family of phosphotransferases with a P-loop motif. J. Biol. Chem. 277, 11362-11367. PubMed
- Lavergne, J.-P., Jault, J.-M. & Galinier, A. (2002) Insights into the functioning of Bacillus subtilis HPr kinase/phosphatase: affinity for its protein substrates and role of cations and phosphate. Biochemistry 41, 6218-6225. PubMed
- Pompeo, F., Granet, Y., Lavergne, J.-P., Grangeasse, C., Nessler, S., Jault, J.-M. & Galinier, A. (2003) Regulation and mutational analysis of the HPr kinase/phosphorylase from Bacillus subtilis. Biochemistry 42, 6762-6771. PubMed
structure analysis
- Márquez, J. A., Hasenbein, S., Koch, B., Fieulaine, S., Nessler, S., Russell, R. B., Hengstenberg, W. & Scheffzek, K. (2002) Structure of the full-length HPr kinase/phosphatase from Staphylococcus xylosus at 1.95 Å resolution: mimicking the product/substrate of the phosphotransfer reactions. Proc. Natl. Acad. Sci. USA 99, 3458-3463. PubMed
- Allen, G.S., Steinhauer, K., Hillen, W., Stülke, J. & Brennan, R.G. (2003) Crystal structure of HPr kinase/phosphatase from Mycoplasma pneumoniae. J. Mol. Biol. 326, 1203-1217. PubMed
- Fieulaine, S., Morera, S., Poncet, S., Mijakovic, I., Galinier, A., Janin, J., Deutscher, J., and Nessler, S. (2002) X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr. Proc Natl Acad Sci U S A 99: 13437-13441. PubMed
HprK as target for antimicrobial compounds
- Ramström, H. et al. (2004) Heterocylic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/ phosphatase from Bacillus subtilis. J. Med. Chem. 47, 2264-2275. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed