Difference between revisions of "RelA"
(→Labs working on this gene/protein) |
(→Biological materials) |
||
| Line 126: | Line 126: | ||
* '''Mutant:''' ''[[relA]]'' mutant {{PubMed|9383190}} - note that ''[[relA]]'' mutants are prone to suppressor mutations in the ''[[ywaC]]'' or ''[[yjbM]]'' loci {{PubMed|18670626}} | * '''Mutant:''' ''[[relA]]'' mutant {{PubMed|9383190}} - note that ''[[relA]]'' mutants are prone to suppressor mutations in the ''[[ywaC]]'' or ''[[yjbM]]'' loci {{PubMed|18670626}} | ||
| − | |||
* '''Expression vector:''' | * '''Expression vector:''' | ||
Revision as of 11:06, 23 October 2014
- Description: GTP pyrophosphokinase (stringent response)
| Gene name | relA |
| Synonyms | |
| Essential | no |
| Product | GTP pyrophosphokinase |
| Function | synthesis and degradation of (p)ppGpp, triggering the stringent response |
| Gene expression levels in SubtiExpress: relA | |
| Metabolic function and regulation of this protein in SubtiPathways: relA | |
| MW, pI | 84 kDa, 6.964 |
| Gene length, protein length | 2202 bp, 734 aa |
| Immediate neighbours | dtd, apt |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 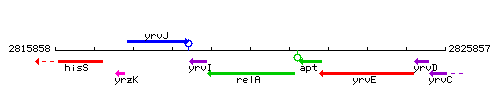
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed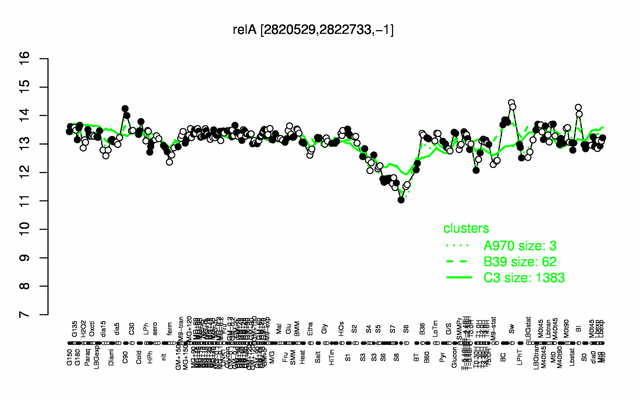
| |
Contents
Categories containing this gene/protein
metabolism of signalling nucleotides
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27600
Phenotypes of a mutant
- requirement for valine PubMed
- a relA sasA sasB triple mutant requires branched chain amino acids, methionine and threonine for growth, the requirement can be suppressed by reduced expression of guaB or inactivation of codY PubMed
- mutation in relA results increased motility and chaining PubMed
- relA mutation increases flagellin production via elevated SigD level PubMed
Database entries
- BsubCyc: BSU27600
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + GTP = AMP + guanosine 3'-diphosphate 5'-triphosphate (according to Swiss-Prot)
- Protein family: relA/spoT family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU27600
- Structure:
- UniProt: O54408
- KEGG entry: [3]
- E.C. number: 2.7.6.5
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: relA mutant PubMed - note that relA mutants are prone to suppressor mutations in the ywaC or yjbM loci PubMed
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Mohamed Marahiel, Marburg University, Germany homepage
Jue D Wang, University of Wisconsin–Madison ResearchGate-Profile
Your additional remarks
References
Qutaiba O Ababneh, Jennifer K Herman
RelA inhibits Bacillus subtilis motility and chaining.
J Bacteriol: 2015, 197(1);128-37
[PubMed:25331430]
[WorldCat.org]
[DOI]
(I p)
Mariangela Tabone, Virginia S Lioy, Silvia Ayora, Cristina Machón, Juan C Alonso
Role of toxin ζ and starvation responses in the sensitivity to antimicrobials.
PLoS One: 2014, 9(1);e86615
[PubMed:24489751]
[WorldCat.org]
[DOI]
(I e)
Allison Kriel, Shaun R Brinsmade, Jessica L Tse, Ashley K Tehranchi, Alycia N Bittner, Abraham L Sonenshein, Jue D Wang
GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes.
J Bacteriol: 2014, 196(1);189-201
[PubMed:24163341]
[WorldCat.org]
[DOI]
(I p)
Ezequiel Wexselblatt, Yaara Oppenheimer-Shaanan, Ilana Kaspy, Nir London, Ora Schueler-Furman, Eylon Yavin, Gad Glaser, Joshua Katzhendler, Sigal Ben-Yehuda
Relacin, a novel antibacterial agent targeting the Stringent Response.
PLoS Pathog: 2012, 8(9);e1002925
[PubMed:23028324]
[WorldCat.org]
[DOI]
(I p)
Yousuke Natori, Kazumi Tagami, Kana Murakami, Sawako Yoshida, Osamu Tanigawa, Yoonsuh Moh, Kenta Masuda, Tetsuya Wada, Shota Suzuki, Hideaki Nanamiya, Yuzuru Tozawa, Fujio Kawamura
Transcription activity of individual rrn operons in Bacillus subtilis mutants deficient in (p)ppGpp synthetase genes, relA, yjbM, and ywaC.
J Bacteriol: 2009, 191(14);4555-61
[PubMed:19447912]
[WorldCat.org]
[DOI]
(I p)
Shuyu Zhang, W G Haldenwang
RelA is a component of the nutritional stress activation pathway of the Bacillus subtilis transcription factor sigma B.
J Bacteriol: 2003, 185(19);5714-21
[PubMed:13129942]
[WorldCat.org]
[DOI]
(P p)
Thomas M Wendrich, Gregor Blaha, Daniel N Wilson, Mohamed A Marahiel, Knud H Nierhaus
Dissection of the mechanism for the stringent factor RelA.
Mol Cell: 2002, 10(4);779-88
[PubMed:12419222]
[WorldCat.org]
[DOI]
(P p)
Takashi Inaoka, Kosaku Takahashi, Mayumi Ohnishi-Kameyama, Mitsuru Yoshida, Kozo Ochi
Guanine nucleotides guanosine 5'-diphosphate 3'-diphosphate and GTP co-operatively regulate the production of an antibiotic bacilysin in Bacillus subtilis.
J Biol Chem: 2003, 278(4);2169-76
[PubMed:12372825]
[WorldCat.org]
[DOI]
(P p)
Takashi Inaoka, Kozo Ochi
RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intracellular GTP.
J Bacteriol: 2002, 184(14);3923-30
[PubMed:12081964]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
S Autret, A Levine, F Vannier, Y Fujita, S J Séror
The replication checkpoint control in Bacillus subtilis: identification of a novel RTP-binding sequence essential for the replication fork arrest after induction of the stringent response.
Mol Microbiol: 1999, 31(6);1665-79
[PubMed:10209741]
[WorldCat.org]
[DOI]
(P p)
T M Wendrich, M A Marahiel
Cloning and characterization of a relA/spoT homologue from Bacillus subtilis.
Mol Microbiol: 1997, 26(1);65-79
[PubMed:9383190]
[WorldCat.org]
[DOI]
(P p)