Difference between revisions of "PrpC"
| Line 56: | Line 56: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15760&redirect=T BSU15760] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 94: | Line 95: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15760&redirect=T BSU15760] | ||
* '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1TXO 1TXO] (from ''Mycobacterium tuberculosis'', 34% identity, 54% similarity) {{PubMed|15530359}} | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1TXO 1TXO] (from ''Mycobacterium tuberculosis'', 34% identity, 54% similarity) {{PubMed|15530359}} | ||
Revision as of 13:41, 2 April 2014
- Description: protein phosphatase
| Gene name | prpC |
| Synonyms | yloO |
| Essential | no |
| Product | protein phosphatase |
| Function | antagonist of PrkC-dependent phosphorylation |
| Gene expression levels in SubtiExpress: prpC | |
| Metabolic function and regulation of this protein in SubtiPathways: prpC | |
| MW, pI | 27 kDa, 4.355 |
| Gene length, protein length | 762 bp, 254 aa |
| Immediate neighbours | yloN, prkC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 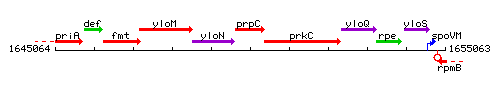
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15760
Phenotypes of a mutant
A prpC mutant is less lytic in late stationary phase. PubMed
Database entries
- BsubCyc: BSU15760
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: A phosphoprotein + H2O = a protein + phosphate (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Proteins dephosphorylated by PrpC
CpgA, EF-Tu, YezB PubMed, HPr PubMed, YkwC PubMed
Extended information on the protein
- Kinetic information:
- Modification:
- Cofactors: divalent cations such as magnesium or manganese
- Effectors of protein activity: inhibited by inorganic phosphate and glycero-2-phosphate PubMed
Database entries
- BsubCyc: BSU15760
- UniProt: O34779
- KEGG entry: [2]
- E.C. number: 3.1.3.16
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: OMG401 (aphA3), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Sandro F F Pereira, Lindsie Goss, Jonathan Dworkin
Eukaryote-like serine/threonine kinases and phosphatases in bacteria.
Microbiol Mol Biol Rev: 2011, 75(1);192-212
[PubMed:21372323]
[WorldCat.org]
[DOI]
(I p)
Original publications