Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' IMP dehydrogenase <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''guaB'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''guaA '' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || IMP dehydrogenase |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || biosynthesis of GMP |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 52 kDa, 6.168 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1464 bp, 488 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[yaaC]]'', ''[[dacA]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB11785]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:guaB_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 38: | Line 38: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | |||
| + | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/guaB.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10073] |
=== Additional information=== | === Additional information=== | ||
| Line 64: | Line 66: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' | + | * '''Modification:''' phosphorylated (STY) [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed], S-cysteinlyation after diamide stress (Cys-308) [http://www.ncbi.nlm.nih.gov/pubmed/17611193 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 78: | Line 80: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P21879 P21879] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU00090] |
* '''E.C. number:''' | * '''E.C. number:''' | ||
| Line 118: | Line 120: | ||
=References= | =References= | ||
| − | # | + | # Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in ''Bacillus subtilis''. ''Proteomics'' '''7:''' 3509-3526. [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] |
| + | # Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in ''Bacillus subtilis''. ''Proteomics'' 7: 3509-3526. [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ||
| + | # Hochgräfe et al. (2007) S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. ''J. Biol. Chem.'' 282: 25981-25985. [http://www.ncbi.nlm.nih.gov/pubmed/17611193 PubMed] | ||
Revision as of 15:23, 4 May 2009
- Description: IMP dehydrogenase
| Gene name | guaB |
| Synonyms | guaA |
| Essential | yes PubMed |
| Product | IMP dehydrogenase |
| Function | biosynthesis of GMP |
| MW, pI | 52 kDa, 6.168 |
| Gene length, protein length | 1464 bp, 488 aa |
| Immediate neighbours | yaaC, dacA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 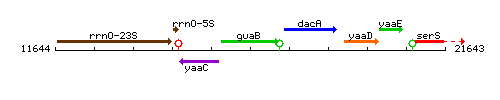
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated (STY) PubMed, S-cysteinlyation after diamide stress (Cys-308) PubMed, PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P21879
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 7: 3509-3526. PubMed
- Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 7: 3509-3526. PubMed
- Hochgräfe et al. (2007) S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J. Biol. Chem. 282: 25981-25985. PubMed