Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' cell | + | * '''Description:''' MreC is a cell shape determining protein and is associated with the [[MreB]] cytoskeleton in ''B. subtilis'' and other rod shaped bacteria.<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''mreC'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | ||
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || cell-shape determining protein | |style="background:#ABCDEF;" align="center"| '''Product''' || cell-shape determining protein | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || cell-shape | + | |style="background:#ABCDEF;" align="center"|'''Function''' || cell-shape determation |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 32 kDa, 6.248 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 870 bp, 290 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[mreD]]'', ''[[mreB]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | ||
| Line 24: | Line 24: | ||
|- | |- | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:mreC_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 37: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Coordinates:''' | + | * '''Coordinates:''' 2859062-2859931 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | + | ''mreC'' is essential under normal conditions [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed]. Depletion of MreC leads to a progressive increase in the width and a decrease in the length of the cell. This shape defect is consistent with a role for ''mreC'' in cell wall synthesis during elongation and has a similar phenotype to other genes with roles in elongation like ''[[rodA]]'' and the redundant gene pair ''[[pbpA]]'' and ''pbpH'' (also known as ''[[ykuA]]''). Electron microscopy of cells depleted of MreC shows regions of the cell where a thick and irregular cell wall has accumulated [http://www.ncbi.nlm.nih.gov/pubmed/12867458 PubMed] and [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed]. ''mreC'' can be deleted provided that 0.5 M sucrose and 20 mM Magnesium is provided in the media, ''mreC'' is therefore conditionally essentail. The phenotype of the ''mreC'' deletion in these conditions is one characterised by extreamly fat and bloated cells that tend to grow in clusters [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed]. |
| + | |||
=== Database entries === | === Database entries === | ||
| Line 47: | Line 48: | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/mreBCD-minCD.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/mreBCD-minCD.html] | ||
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10327] |
| − | === | + | |
| + | = Additional information= | ||
| + | |||
| + | ===Function=== | ||
| + | |||
| + | MreC functions in cell wall synthesis by, together with the [[MreB]] cytoskeleton, localizing the cell wall synthetic machinery to the correct part of the cell. MreC therefore ensures that the cell wall is made in the correct way to maintain the proper shape of the cell. | ||
| + | |||
| + | ===MreC in other organisms=== | ||
| + | |||
| + | MreC has been studied in other organisms where it has been shown to be important in cell shape determination. | ||
| + | |||
| + | *''Escherishia coli'' [http://www.ncbi.nlm.nih.gov/pubmed/15612918 PubMed] [http://www.ncbi.nlm.nih.gov/pubmed/17993535 PubMed] | ||
| + | *''Caulobacter cresentus'' [http://www.ncbi.nlm.nih.gov/pubmed/16344480 PubMed] [http://www.ncbi.nlm.nih.gov/pubmed/16344481 PubMed] | ||
| + | *''Rhodobacter spheroides'' [http://www.ncbi.nlm.nih.gov/pubmed/16484180 PubMed] | ||
| + | *''Streptomyces coelicolor'' [http://www.ncbi.nlm.nih.gov/pubmed/10954092 PubMed] | ||
| Line 56: | Line 71: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' None/ structural protein |
| − | * '''Protein family:''' | + | * '''Protein family:''' COG1793 |
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' None |
=== Extended information on the protein === | === Extended information on the protein === | ||
| − | * '''Kinetic information:''' | + | * '''Kinetic information:''' None |
| − | * '''Domains:''' | + | * '''Domains:''' Intracellular N-terminus, transmembrane domain, Coiled coil domain and C-terminal beta-sheet domain. |
| − | * '''Modification:''' | + | * '''Modification:''' None |
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' None |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' [[ | + | * '''Interactions:''' Interacts with [[MreD]], and a subset of the PBPs [http://www.ncbi.nlm.nih.gov/pubmed/17427287 PubMed] |
| − | * '''Localization:''' | + | * '''Localization:''' GFP-MreC localises to the cell membrane in a helical pattern [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed]. |
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:-''' |
| + | ** 2J5U: MreC from ''Lysteria monocytogenes'' [http://www.ncbi.nlm.nih.gov/pubmed/17427287 PubMed] | ||
| + | ** 2QF4: MreC monomer from ''Streptococcus pneumoniae'' [http://www.ncbi.nlm.nih.gov/pubmed/17707860 PubMed] | ||
| + | ** 2QF5: MreC dimer from ''Streptococcus pneumoniae'' [http://www.ncbi.nlm.nih.gov/pubmed/17707860 PubMed] | ||
| − | * '''Swiss prot entry:''' | + | * '''Swiss prot entry:''' Q01466 |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU28020] |
* '''E.C. number:''' | * '''E.C. number:''' | ||
| Line 104: | Line 122: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' A non-polar inframe deletion strain named 3481 and a xylose dependent conditional mutant named 3461 is avaliable from the [[Errington]] lab [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed]. |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 110: | Line 128: | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| − | * '''GFP fusion:''' | + | * '''GFP fusion:''' A functional N-terminal GFP fusion has been made where the fusion protein is the only copy of the gene in the cell: strain 3417 [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed]. |
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' |
| − | * '''Antibody:''' | + | * '''Antibody:''' antisera raised in rabit is avaliable from the [[Errington]] lab. |
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| Line 123: | Line 141: | ||
=Your additional remarks= | =Your additional remarks= | ||
| + | |||
| + | ''mreC'' is an abbreviation of murein region e, gene C | ||
=References= | =References= | ||
| − | # | + | # Lee JC & Stewart GC (2003) Essential nature of the ''mreC'' determinant of ''Bacillus subtilis'' ''Journal of Bacteriology'' '''185(15)''': 4490-8. [http://www.ncbi.nlm.nih.gov/pubmed/12867458 PubMed] |
| − | # | + | # Leaver M & Errington J (2005) Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in ''Bacillus subtilis'' ''Molecular Microbiology'' '''57(5)''': 1196-209 [http://www.ncbi.nlm.nih.gov/pubmed/16101995 PubMed] |
| − | # | + | # Kruse T, Bork-Jensen J & Gerdes K (2005) The morphogenetic MreBCD proteins of ''Escherichia coli'' form an essential membrane-bound complex ''Molecular Microbiology'' '''55(1)''': 78-89 [http://www.ncbi.nlm.nih.gov/pubmed/15612918 PubMed] |
| − | # | + | #Bendezú FO & de Boer PA (2008) Conditional lethality, division defects, membrane involution, and endocytosis in ''mre'' and ''mrd'' shape mutants of ''Escherichia coli'' ''Journal of Bacteriology'' '''190(5)''': 1792-811 [http://www.ncbi.nlm.nih.gov/pubmed/17993535 PubMed] |
| − | # | + | #Divakaruni AV, Loo RR, Xie Y, Loo JA & Gober JW (2005) The cell-shape protein MreC interacts with extracytoplasmic proteins including cell wall assembly complexes in ''Caulobacter crescentus'' ''PNAS'' '''102(51)''': 18602-7 [http://www.ncbi.nlm.nih.gov/pubmed/16344480 PubMed] |
| + | #Dye NA, Pincus Z, Theriot JA, Shapiro L & Gitai Z (2005) Two independent spiral structures control cell shape in Caulobacter ''PNAS'' 102(51): 18608-13. | ||
| + | #Slovak PM, Porter SL & Armitage JP (2006) Differential localization of Mre proteins with PBP2 in ''Rhodobacter sphaeroides'' ''Journal of Bacteriology'' '''188(5)''': 1691-700 | ||
| + | #Burger A, Sichler K, Kelemen G, Buttner M & Wohlleben W (2000) Identification and characterization of the ''mre'' gene region of ''Streptomyces coelicolor'' A3(2). Molecular and General Genetics '''263(6)''': 1053-60 | ||
| + | # van den Ent F, Leaver M, Bendezu F, Errington J, de Boer P, Löwe J. (2006) Dimeric structure of the cell shape protein MreC and its functional implications. ''Mol Microbiol.'' '''Dec;62(6):''' 1631-42. [http://www.ncbi.nlm.nih.gov/pubmed/17427287 PubMed] | ||
Revision as of 14:06, 15 April 2009
- Description: MreC is a cell shape determining protein and is associated with the MreB cytoskeleton in B. subtilis and other rod shaped bacteria.
| Gene name | mreC |
| Synonyms | |
| Essential | yes PubMed |
| Product | cell-shape determining protein |
| Function | cell-shape determation |
| MW, pI | 32 kDa, 6.248 |
| Gene length, protein length | 870 bp, 290 aa |
| Immediate neighbours | mreD, mreB |
| Hier soll was neues rein | |
Genetic context 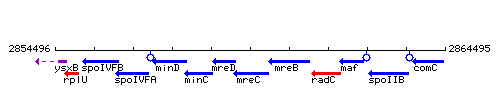
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates: 2859062-2859931
Phenotypes of a mutant
mreC is essential under normal conditions PubMed. Depletion of MreC leads to a progressive increase in the width and a decrease in the length of the cell. This shape defect is consistent with a role for mreC in cell wall synthesis during elongation and has a similar phenotype to other genes with roles in elongation like rodA and the redundant gene pair pbpA and pbpH (also known as ykuA). Electron microscopy of cells depleted of MreC shows regions of the cell where a thick and irregular cell wall has accumulated PubMed and PubMed. mreC can be deleted provided that 0.5 M sucrose and 20 mM Magnesium is provided in the media, mreC is therefore conditionally essentail. The phenotype of the mreC deletion in these conditions is one characterised by extreamly fat and bloated cells that tend to grow in clusters PubMed.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
Function
MreC functions in cell wall synthesis by, together with the MreB cytoskeleton, localizing the cell wall synthetic machinery to the correct part of the cell. MreC therefore ensures that the cell wall is made in the correct way to maintain the proper shape of the cell.
MreC in other organisms
MreC has been studied in other organisms where it has been shown to be important in cell shape determination.
- Escherishia coli PubMed PubMed
- Caulobacter cresentus PubMed PubMed
- Rhodobacter spheroides PubMed
- Streptomyces coelicolor PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: None/ structural protein
- Protein family: COG1793
- Paralogous protein(s): None
Extended information on the protein
- Kinetic information: None
- Domains: Intracellular N-terminus, transmembrane domain, Coiled coil domain and C-terminal beta-sheet domain.
- Modification: None
- Cofactor(s): None
- Effectors of protein activity:
- Localization: GFP-MreC localises to the cell membrane in a helical pattern PubMed.
Database entries
- Structure:-
- Swiss prot entry: Q01466
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: A non-polar inframe deletion strain named 3481 and a xylose dependent conditional mutant named 3461 is avaliable from the Errington lab PubMed.
- Expression vector:
- lacZ fusion:
- GFP fusion: A functional N-terminal GFP fusion has been made where the fusion protein is the only copy of the gene in the cell: strain 3417 PubMed.
- two-hybrid system:
- Antibody: antisera raised in rabit is avaliable from the Errington lab.
Labs working on this gene/protein
Jeff Errington, Newcastle University, UK homepage
Peter Graumann, Freiburg University, Germany homepage
Your additional remarks
mreC is an abbreviation of murein region e, gene C
References
- Lee JC & Stewart GC (2003) Essential nature of the mreC determinant of Bacillus subtilis Journal of Bacteriology 185(15): 4490-8. PubMed
- Leaver M & Errington J (2005) Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis Molecular Microbiology 57(5): 1196-209 PubMed
- Kruse T, Bork-Jensen J & Gerdes K (2005) The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex Molecular Microbiology 55(1): 78-89 PubMed
- Bendezú FO & de Boer PA (2008) Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli Journal of Bacteriology 190(5): 1792-811 PubMed
- Divakaruni AV, Loo RR, Xie Y, Loo JA & Gober JW (2005) The cell-shape protein MreC interacts with extracytoplasmic proteins including cell wall assembly complexes in Caulobacter crescentus PNAS 102(51): 18602-7 PubMed
- Dye NA, Pincus Z, Theriot JA, Shapiro L & Gitai Z (2005) Two independent spiral structures control cell shape in Caulobacter PNAS 102(51): 18608-13.
- Slovak PM, Porter SL & Armitage JP (2006) Differential localization of Mre proteins with PBP2 in Rhodobacter sphaeroides Journal of Bacteriology 188(5): 1691-700
- Burger A, Sichler K, Kelemen G, Buttner M & Wohlleben W (2000) Identification and characterization of the mre gene region of Streptomyces coelicolor A3(2). Molecular and General Genetics 263(6): 1053-60
- van den Ent F, Leaver M, Bendezu F, Errington J, de Boer P, Löwe J. (2006) Dimeric structure of the cell shape protein MreC and its functional implications. Mol Microbiol. Dec;62(6): 1631-42. PubMed