Difference between revisions of "DivIVA"
(→Extended information on the protein) |
(→Extended information on the protein) |
||
| Line 63: | Line 63: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' The first 60 amino acids constitute a lipid binding domain. Multimerisation involves two coiled-coil motifs, one in the lipid binding domain, and the other one being present in the helical C-terminal domain. | + | * '''Domains:''' The first 60 amino acids constitute a lipid binding domain. [http://www.ncbi.nlm.nih.gov/sites/entrez/19478798 PubMed] |
| + | Multimerisation involves two coiled-coil motifs, one in the lipid binding domain, and the other one being present in the helical C-terminal domain. | ||
* '''Modification:''' The Mycobacterium DivIVA homologue Wag31 is phosphorylated at T73. | * '''Modification:''' The Mycobacterium DivIVA homologue Wag31 is phosphorylated at T73. | ||
Revision as of 14:59, 14 July 2009
- Description: cell-division initiation protein (septum placement)
| Gene name | divIVA |
| Synonyms | ylmJ |
| Essential | no |
| Product | cell-division initiation protein |
| Function | septum placement |
| MW, pI | 19 kDa, 4.846 |
| Gene length, protein length | 492 bp, 164 aa |
| Immediate neighbours | ylmH, ileS |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 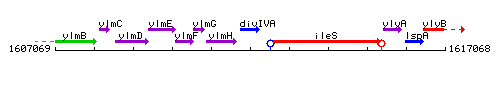
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU15420
Phenotypes of a mutant
Deletion of divIVA leads to filamentation and polar divisions that in turn cause a minicell phenotype. A divIVA mutant has a severe sporulation defect.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: DivIVA is required for polar localisation of MinCD via MinJ. It also recruits RacA to the distal pole of the prespore.
- Protein family: gpsB family (according to Swiss-Prot)
- Paralogous protein(s): GpsB
Extended information on the protein
- Kinetic information:
- Domains: The first 60 amino acids constitute a lipid binding domain. PubMed
Multimerisation involves two coiled-coil motifs, one in the lipid binding domain, and the other one being present in the helical C-terminal domain.
- Modification: The Mycobacterium DivIVA homologue Wag31 is phosphorylated at T73.
- Cofactor(s):
- Effectors of protein activity:
- Localization: DivIVA forms a ring underneath the invaginating membrane at the site of cell division and is enriched at both cell poles.
Database entries
- Structure:
- Swiss prot entry: P71021
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: one gene cistron
- Additional information:
Biological materials
- Mutant: divIVA::tet available from the Hamoen Lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Centre for Bacterial Cell Biology, Newcastle upon Tyne, United Kingdom [4]
Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
Your additional remarks
References